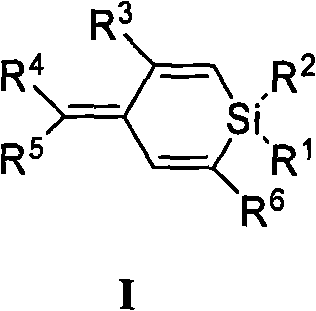
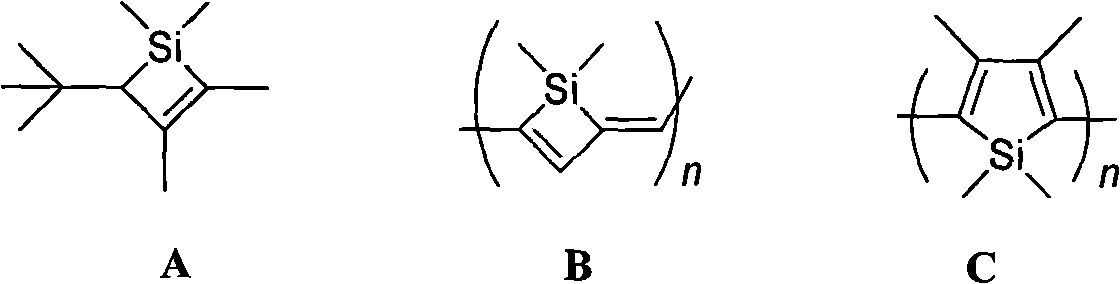
Polysubstituted silacyclohexadiene and synthetic method thereof
A silacyclohexadiene, multi-substituted technology, which is applied in chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, organic chemistry, etc., can solve problems such as application research, and achieve high separation yields , convenient for industrialization, and the effect of a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Ia(R 1 = R 2 = Me,R 3 =COOMe,R 4 = Ph, R 5 = H, R 6 =Ph): Synthesis of (4Z)-methyl 4-benzylidene-1,4-dihydro-1,1-dimethyl-6-phenyl-silacyclohexadiene-3-carbonate
[0035] Add 1 mmol of polysubstituted silacyclobutene IIa (R 1 = R 2 = Me,R 4 = Ph, R 5 = H, R 6 =Ph)[(2Z)-2-benzylidene-1,2-dihydro-1,1-dimethyl-4-phenylsilacyclobutene], 1 mmol propiolate, 1% equivalent Pd(PPh 3 ) 4 and 5mL of toluene solvent, heated to reflux, and magnetically stirred for 3 hours. Concentrate after the reaction, decolorize and separate on a silica gel column, and use a mixed solvent of petroleum ether: ether = 10:1 as eluent to obtain the pure product (4Z)-methyl 4-benzylidene-1,4-dihydro-1, 1-Dimethyl-6-phenyl-silacyclohexadiene-3-carbonate 0.315 g (purity>98%, colorless liquid), isolated yield 91%. The NMR, IR and high-resolution mass spectrometry data of the compound are as follows: 1 H NMR (300MHz, CDCl 3 , Me 4 Si): δ=0.35(s, 6H), 3.09(s, 3H), 6.98(s, 1H), 7.23-7.36(m,...
Embodiment 2
[0037] Ib(R 1 = R 2 = Ph, R 3 =COOMe,R 4 = Ph, R 5 = H, R 6 =Ph) synthesis:
[0038] Add 1 mmol of polysubstituted silacyclobutene IIb (R 1 = R 2 = Ph, R 4 = Ph, R 5 = H, R 6 =Ph), 1 mmol propiolate, 1% equivalent of Pd (PPh 3 ) 4 and 5mL of toluene solvent, heated to reflux, and magnetically stirred for 3 hours. Concentrate after the reaction, decolorize and separate on a silica gel column, and use a mixed solvent of petroleum ether: ether=10:1 as an eluent to obtain the pure product multi-substituted silacyclohexadiene Ib (R 1 = R 2 = Me,R 3 =COOMe,R 4 = Ph, R 5 =H) 0.418 g (purity>98%, colorless liquid), isolated yield 89%. The NMR, IR and high-resolution mass spectrometry data of the compound are as follows: 1 H NMR (CDCl 3 , Me 4 Si): δ=3.10(s, 3H, CH 3 ), 7.16-7.58 (m, 23H, CH); 13 C NMR (CDCl 3 , Me 4 Si): δ=51.6, 126.8, 127.1, 128.1, 128.2, 128.3, 128.5, 129.1, 123.0, 133.2, 133.5, 135.4, 135.7, 137.6, 137.7, 140.6, 142.7, 145.8, 147.3, 168.6: I...
Embodiment 3
[0040] Ic(R 1 = R 2 = Me,R 3 = COPh,R 4 = Ph, R 5 = H, R 6 =Ph): Synthesis of ((4Z)-4-benzylidene-1,4-dihydro-1,1-dimethyl-6-phenylsilacyclohexadien-3-yl)benzophenone
[0041] Add 1 mmol of polysubstituted silacyclobutene IIc (R 1 = R 2 = Me,R 4 = Ph, R 5 = H, R 6 =Ph)[(2Z)-1,2-dihydro-1,1-dimethyl-4-phenyl-2-((E)-1-phenyl-2-propylhex-2-enyl Methylene) silacyclobutene], 1.5 mmol alkynyl benzophenone, 1% equivalent of Pd (PPh 3 ) 4 and 5mL of toluene solvent, heated to reflux, and magnetically stirred for 1 hour. Concentration after the reaction, decolorization and separation on a silica gel column, using a mixed solvent of petroleum ether: ether = 10: 1 as eluent, the obtained pure product ((4Z)-4-benzylidene-1,4-dihydro-1, 0.274 g of 1-dimethyl-6-phenylsilacyclohexadien-3-yl)benzophenone (purity>98%, colorless solid), isolated yield 70%. The NMR and IR high-resolution mass spectrometry data of the compound are as follows: 1 H NMR (300MHz, CDCl 3 , Me 4 Si): δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com