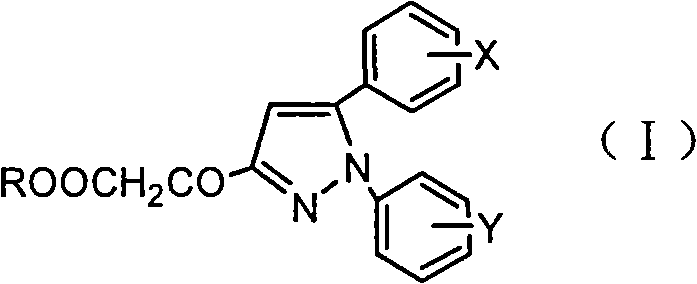
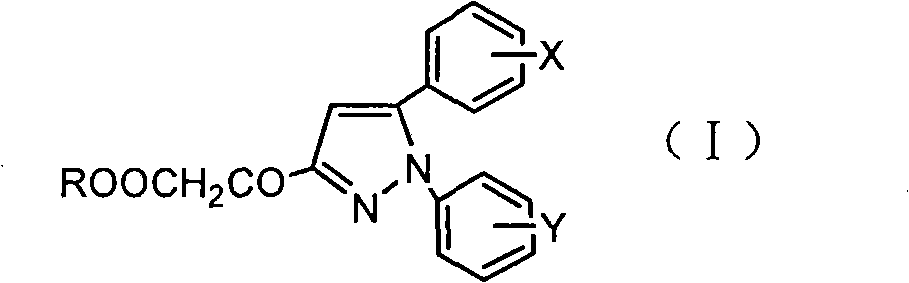
Pyrazoleoxy acetic acid compounds, preparation method and use
A compound, the technology of oxyacetoxypyrazole, applied in the preparation, the application of fungicides, the field of pyrazoloxyacetic acid compounds, can solve the problems of many times of re-infection, difficult to control plant diseases, epidemic diseases and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
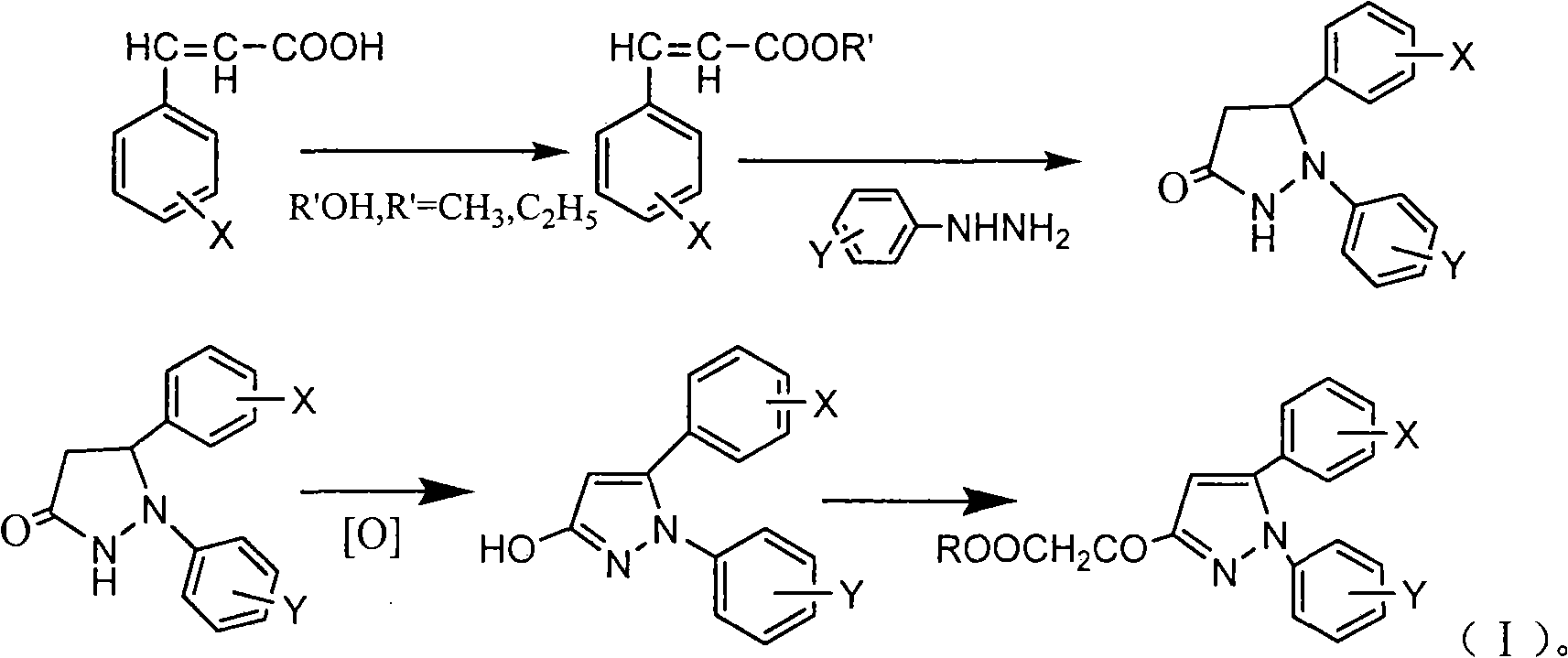
Method used
Image
Examples
Embodiment 1
[0021] Under stirring, add 50ml of anhydrous methanol and 0.3g of p-toluenesulfonic acid to 4-methoxycinnamic acid (0.05mol). After reflux reaction for 2h, methanol is distilled off under reduced pressure to obtain methyl 4-methoxycinnamate 8.82g; add methyl 4-methoxycinnamate and 50ml n-butanol to the newly prepared 28% sodium methoxide solution under stirring, react at 100°C for 4h, then add 8ml phenylhydrazine, continue to heat and reflux for 24h Afterwards, cool and filter to obtain 10.11 g of 1-phenyl-5-(4-methoxyphenyl)-3-pyrazolidinone; this compound was dissolved in N,N-dimethylformamide solution, Air was blown in, reacted at 100°C for 1 h, cooled to room temperature, and filtered to obtain 8.92 g of 1-phenyl-5-(4-methoxyphenyl)-3-hydroxypyrazole with a yield of 66.9%; Add 4.42g of hydroxypyrazole to 100ml of water, drop an equivalent amount of ethyl bromoacetate, react at 100°C for 2 hours, extract with ethyl acetate, dry over anhydrous magnesium sulfate, filter, and ...
Embodiment 2
[0023] Under stirring, add 100ml of anhydrous methanol and 0.5g of p-toluenesulfonic acid to 3,4,5-trimethoxycinnamic acid (0.1mol), after reflux for 2h, distill under reduced pressure to obtain 4-methoxycinnamic acid Methyl ester 19.68g; Add methyl 3,4,5-trimethoxycinnamate and 50ml n-butanol to the newly prepared 28% sodium methoxide solution under stirring, react at 100°C for 4h, then add 10ml phenylhydrazine , after continuing to heat and reflux for 24h, cool and filter to obtain 24.96g of 1-phenyl-5-(3,4,5-trimethoxyphenyl)-3-pyrazolidinone; this compound was dissolved in N, In N-dimethylformamide solution, air was blown in, reacted at 100°C for 1h, cooled to room temperature, and filtered to obtain 1-phenyl-5-(3,4,5-trimethoxyphenyl)- 22.61g of 3-hydroxypyrazole, yield 69.3%; take 11.31g of hydroxypyrazole and add it to 100ml of water, add dropwise the same amount of bromoacetic acid, react at 100°C for 2h, extract with ethyl acetate, anhydrous magnesium sulfate After d...
Embodiment 3
[0025] Under stirring, add 20ml of absolute ethanol and 0.3g of p-toluenesulfonic acid to 4-bromocinnamic acid (0.05mol), after reflux reaction for 2h, distill under reduced pressure to obtain 11.29g of ethyl 4-bromocinnamate; under stirring Add ethyl 4-bromocinnamate and 50ml of n-butanol to the newly prepared 28% sodium methoxide solution, react at 100°C for 4 hours, then add 6.55g of p-fluorophenylhydrazine, continue to heat and reflux for 24 hours, cool and filter , to obtain 1-(4-fluorophenyl)-5-(4-bromophenyl)-3-pyrazolidinone 12.1g; this compound was dissolved in N,N-dimethylformamide solution, blown into Air, reacted at 100°C for 1 hour, cooled to room temperature, and filtered to obtain 10.92 g of 1-(4-fluorophenyl)-5-(4-bromophenyl)-3-hydroxypyrazole with a yield of 65.3%; Take 5.32g of hydroxypyrazole and add it to 100ml of water, add an equivalent amount of chloroacetic acid dropwise, react at 100°C for 2 hours, extract with ethyl acetate, dry over anhydrous magnes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com