Separation and purification method of cefamandole nafate and preparation of cefathiamidine freeze-dried injectable powder
A technology for the separation and purification of cefamandole sodium, which is applied in freeze-dried transportation, medical preparations containing active ingredients, organic chemistry, etc. It can solve problems such as poor clarity, low purity, and poor stability, and achieve improved purity , the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
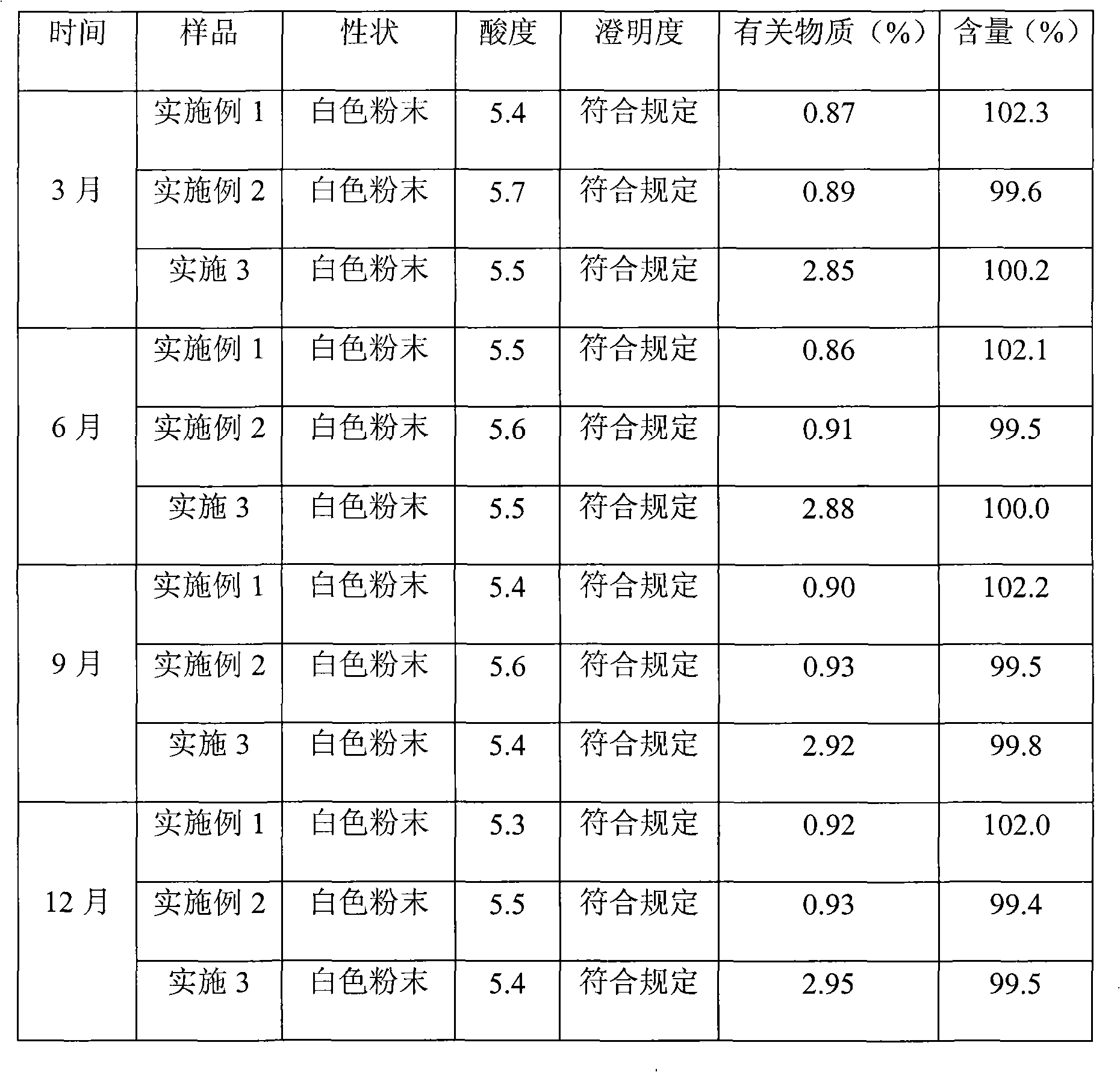
Embodiment 1
[0018] (1) Cefamandole sodium raw material loading amount 200mg, purity 95.8%. Solvent system: chloroform, ethyl acetate, methanol, water volume ratio 2:2.5:1.8:1 (volume ratio), column volume: 200ml, rotating speed 800rpm, upper phase is stationary phase, lower phase is mobile phase, flow rate 2ml / min, stationary phase retention: 52%.
[0019] Prepare a solvent system according to the volume ratio of chloroform, ethyl acetate, methanol, and water in a ratio of 2:2.5:1.8:1, let it stand for stratification, separate the upper and lower phases, take the upper phase as the stationary phase, and the lower phase as the mobile phase. Make the high-speed countercurrent chromatograph column full of stationary phase, then make the main engine rotate clockwise, then pump the mobile phase into the column, dissolve the cefamandole sodium raw material with the lower phase solvent, and then inject the sample through the injection valve. The spectrum receives the target components.
[002...
Embodiment 2
[0026] Get cefamandole sodium raw material, operation step is by embodiment 1, and difference is: in step (1), solvent system: the volume ratio of chloroform, ethyl acetate, methyl alcohol, water is 1.5: 1.8: 1: 1 , solvent system in step (2): chloroform: ethyl acetate: methanol: the volume ratio of water is 1.0: 0.8: 1.3: 1, solvent system in step (3): chloroform: ethyl acetate: methanol: The volume ratio of water is 0.8:0.5:1.0:1. After purification, the purity is 99.3% as detected by high performance liquid chromatography. Aseptic subpackaging to obtain a sterile preparation of cefamandole sodium freeze-dried powder for injection.
Embodiment 3
[0028] Get cefamandole sodium raw material, operation step is by embodiment 1, and difference is: in step (1), solvent system: the volume ratio of chloroform, ethyl acetate, methyl alcohol, water is 3.0: 3.5: 2.5: 1 , solvent system in step (2): chloroform: ethyl acetate: methanol: the volume ratio of water is 2.5: 2.0: 1.5: 1, solvent system in step (3): chloroform: ethyl acetate: methanol: The volume ratio of water is 0.5:1.0:0.7:1. After purification, the purity detected by high performance liquid chromatography was 97.2%. Aseptic subpackaging to obtain a sterile preparation of cefamandole sodium freeze-dried powder for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com