Berbamine derivative and application of salt thereof
A technology of berbamine and its derivatives, applied in the application field of compounds and their derivatives, to achieve strong anti-leukemia and solid tumor activity, and light toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
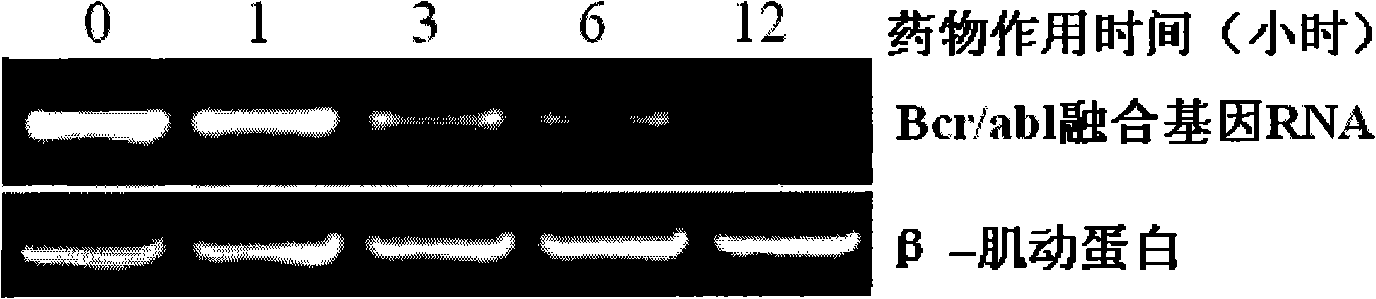
[0045] Example 1: Comparison of newly synthesized berbamine derivative 4-chlorobenzoyl berbamine with known berbamine derivative 4ethoxybutyl berbamine and berbamine anti-human leukemia activity
[0046] (1) Experimental materials
[0047] Cell line: human K562 leukemia cell line was purchased from Shanghai Cell Bank, Chinese Academy of Sciences.
[0048] Reagents: The standard berbamine was purchased from China National Institute of Pharmaceutical and Biological Products, and the berbamine derivatives 4-chlorobenzoyl berbamine and 4-ethoxybutyl berbamine were synthesized by ourselves.
[0049] Instruments: cell incubator, microplate reader
[0050] (2) Experimental method
[0051] Take 8,000 well-grown leukemia cells and inoculate them into wells of a 96-well cell culture plate. The culture medium is 1640 cell culture medium containing 10% fetal bovine serum. Drugs of different concentrations were added, mixed evenly, and placed in a carbon dioxide (5% CO2) cell incubator...
Embodiment 2
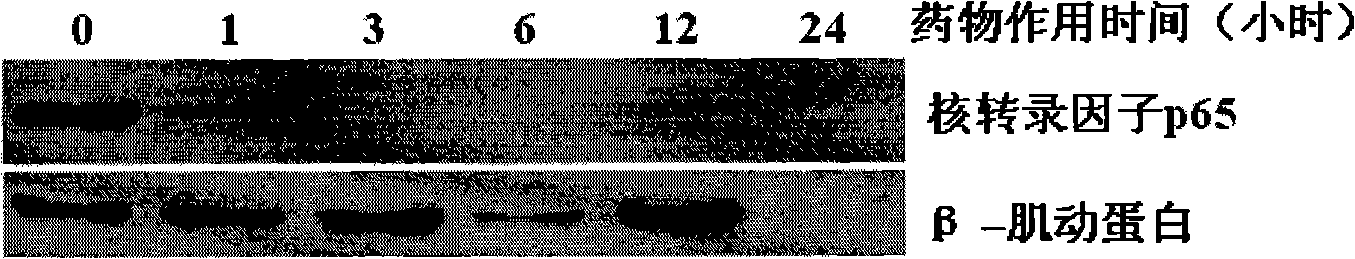
[0054] Embodiment 2: Comparison of anti-human leukemia activity of newly synthesized berbamine derivative 4-chlorobenzoyl berbamine and its hydrochloride-hydrochloride 4-chlorobenzoyl berbamine
[0055] (1) Experimental materials
[0056] Cell line: human K562 leukemia cell line was purchased from Shanghai Cell Bank, Chinese Academy of Sciences.
[0057] Reagent: berbamine derivative 4-chlorobenzoyl berbamine and its hydrochloride - 4-chlorobenzoyl berbamine hydrochloride is synthesized by ourselves.
[0058] Instruments: cell incubator, microplate reader
[0059] (2) Experimental method
[0060] Take 8,000 well-grown leukemia cells and inoculate them into wells of a 96-well cell culture plate. The culture medium is 1640 cell culture medium containing 10% fetal bovine serum. Drugs of different concentrations were added, mixed evenly, and placed in a carbon dioxide (5% CO2) cell incubator at 37° C. for 48 hours. The concentration of viable cells was then determined by the ...
Embodiment 3
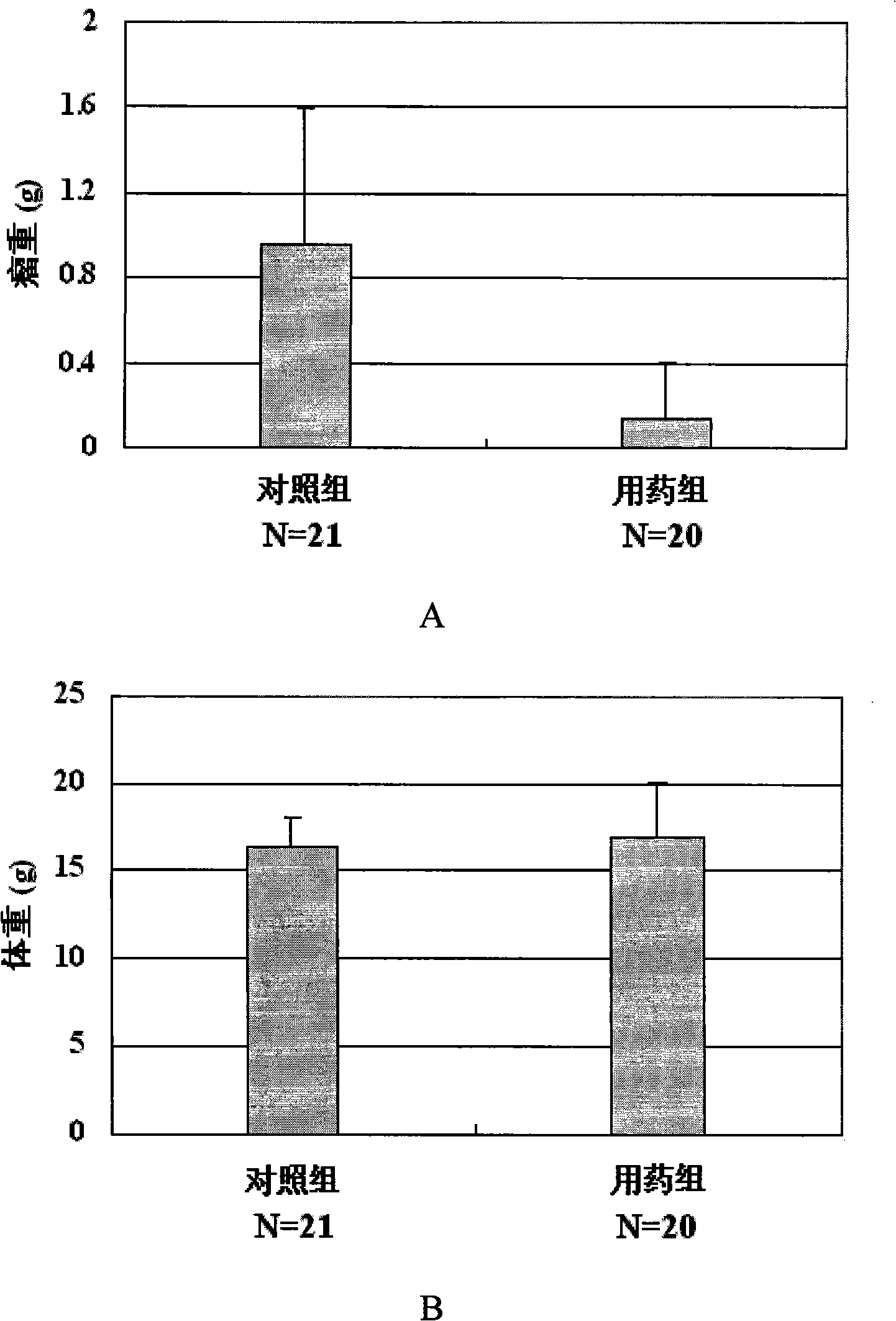
[0063] Embodiment 3: newly synthesized berbamine derivatives 3,4,5-trimethoxybenzoyl berbamine, 4-methoxybenzoyl berbamine and 2-methylbenzoyl berbamine anti Human leukemia activity assay
[0064] (1) Experimental materials
[0065] Cell line: human K562 leukemia cell line was purchased from Shanghai Cell Bank, Chinese Academy of Sciences.
[0066] Reagents: 3,4,5-trimethoxybenzoyl berbamine, 4-methoxybenzoyl berbamine and 2-methylbenzoyl berbamine were synthesized by ourselves.
[0067] Instruments: cell incubator, microplate reader
[0068] (2) Experimental method
[0069] Take 8,000 well-grown leukemia cells and inoculate them into wells of a 96-well cell culture plate. The culture medium is 1640 cell culture medium containing 10% fetal bovine serum. After cultivating at 37° C. in a carbon dioxide (5% CO 2 ) cell incubator for 24 hours, add different concentrations of medicines, mix well, and continue to put in a carbon dioxide (5% CO 2 ) cell incubator at 37° C. to cu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com