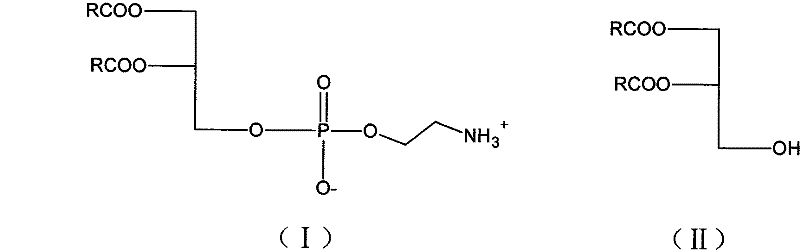
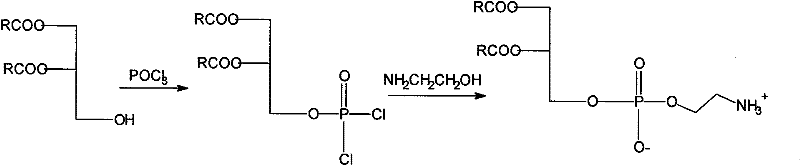
Novel method for synthesizing bi-axungia acyl-phosphatidylethanolamine
A technology of stearoyl phosphatidylethanolamine and palmitoyl phosphatidylethanolamine, which is applied in the direction of phosphorus organic compounds, etc., to achieve the effects of simplifying the process, reducing production costs, and simple synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 2: Preparation of dipalmitoylphosphatidylethanolamine
[0020] Dissolve 2g (0.0035mol) dipalmitoylglyceride, 1.34g (0.0087mol) phosphorus oxychloride, 1.21g (0.012mol) triethylamine in 24 chloroform solution, control the temperature at 35 degrees, then react for 2h, react After stopping, filter and concentrate the filtrate to obtain a concentrated solution, then react with 0.55g (0.009mol) ethanolamine and 2.42g (0.024mol) triethylamine in 20ml tetrahydrofuran solution at a temperature of 20 degrees, react for 15min, filter, and concentrate the filtrate. get crude. The crude product was purified with cyclohexane to obtain 2.1 g of dipalmitoylphosphatidylethanolamine. 1 H-NMR (CDCl 3 , δppm): 0.88 (6H, t), 1.23-1.27 (48H, m), 1.64 (4H, d), 2.27-2.32 (4H, m), 3.22-3.26 (2H, m), 4.1 (2H, d ), 4.12-4.19 (3H, m), 4.36-4.38 (1H, m), 5.23 (1H, s), 8.33 (3H, s).
Embodiment 2
[0021] Embodiment 3: Preparation of Dimyrisyl Phosphatidylethanolamine
[0022] Dissolve 2g (0.004mol) of digicolithylglyceride, 1.23g (0.008mol) of phosphorus oxychloride, and 1.01g (0.01mol) of triethylamine in 14ml of trichloroethylene solution at a temperature of 20 degrees, then react for 4 hours, and react After stopping, filter and concentrate the filtrate to obtain a concentrate, then react with 0.49g (0.008mol) ethanolamine and 2.02g (0.02mol) triethylamine in 12ml of chloroform solution at a temperature of 15°C for 30min, filter and concentrate the filtrate. get crude. The crude product was refined with petroleum ether to obtain 1.96 g of dimyrisylphosphatidylethanolamine. 1 H-NMR (CDCl 3 , δppm): 0.89 (6H, t), 1.24-1.27 (40H, m), 1.60 (4H, d), 2.20-2.32 (4H, m), 3.21-3.23 (2H, m), 4.0 (2H, d ), 4.10-4.16 (3H, m), 4.36-4.38 (1H, m), 5.23 (1H, s), 8.33 (3H, s).
Embodiment 3
[0023] Embodiment 4: Preparation of dipalmitoylphosphatidylethanolamine
[0024] Dissolve 2g (0.0035mol) of dipalmitoylglyceride, 0.97g (0.0063mol) of phosphorus oxychloride, and 0.71g (0.007mol) of triethylamine in 10ml of trichloroethylene solution and 4ml of dichloromethane, and control the temperature at 0 degree, then reacted for 5.5h, after the reaction stopped, filtered, and the filtrate was concentrated to obtain a concentrated solution, which was then reacted with 0.43g (0.007mol) ethanolamine and 1.01g (0.01mol) triethylamine in 14ml dichloromethane solution, and the temperature was 5 degree, reacted for 35min, filtered, and the filtrate was concentrated to obtain a crude product. The crude product was refined with n-hexane to obtain 2.08 g of dipalmitoylphosphatidylethanolamine. HPLC: 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com