Soluble preparation of bone morphogenesis protein-7 and application thereof
A morphogenic protein and soluble technology, which is applied in the direction of peptide/protein components, medical preparations containing active ingredients, bone diseases, etc., can solve the problems of low solubility, difficult to dissolve, hinder the development of other dosage forms and clinical application, etc., to achieve The effect of high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Take 300 mg of recombinant human rhBMP-7 (purity up to 95%) expressed by CHO of purified mammalian cells and add it to 0.3M / L L-arginine solution (adjust pH=6.5), and use the same concentration of L-arginine The amino acid solution was fixed to 1000 milliliters, so that the final concentration of BMP-7 was 0.3 mg / ml, and then after depyrogenation and filter sterilization, it was subpackaged into bottled injections (3 mg / bottle) of clinically commonly used doses.
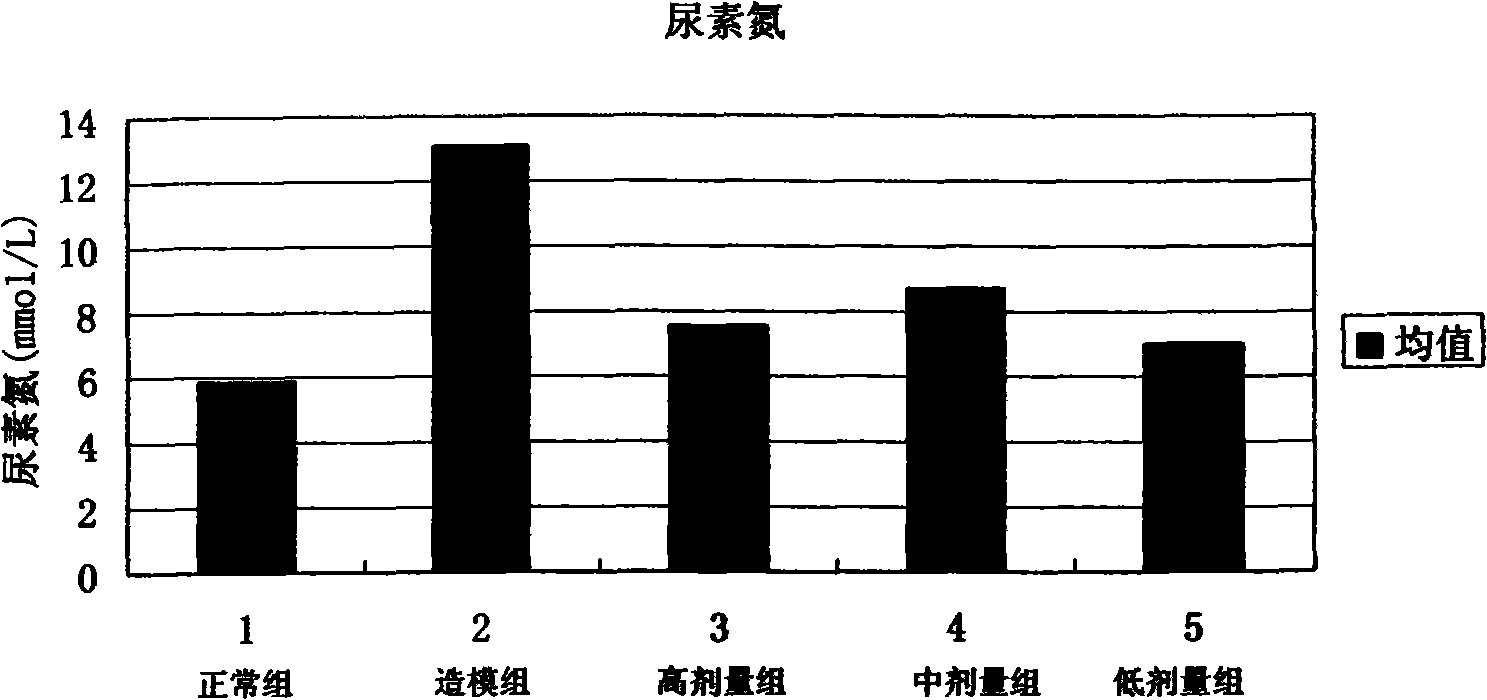
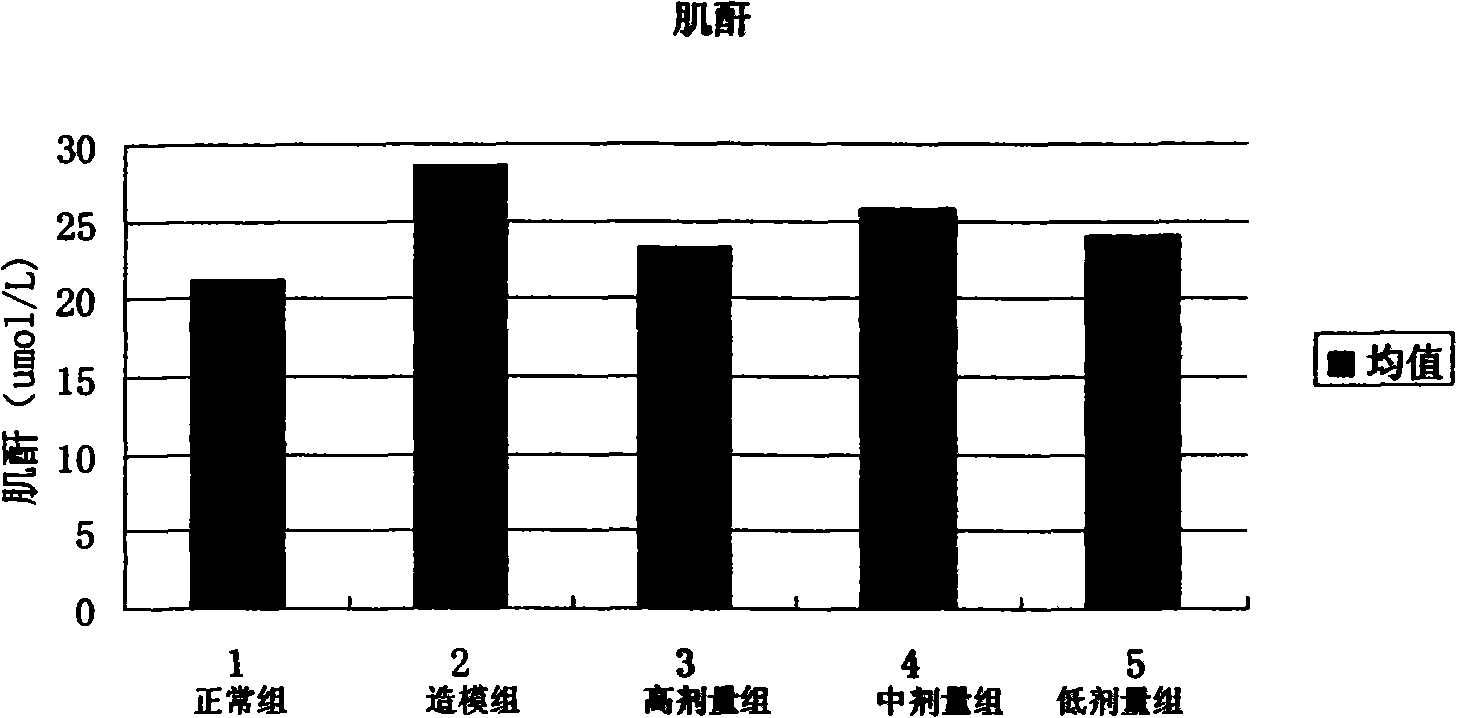
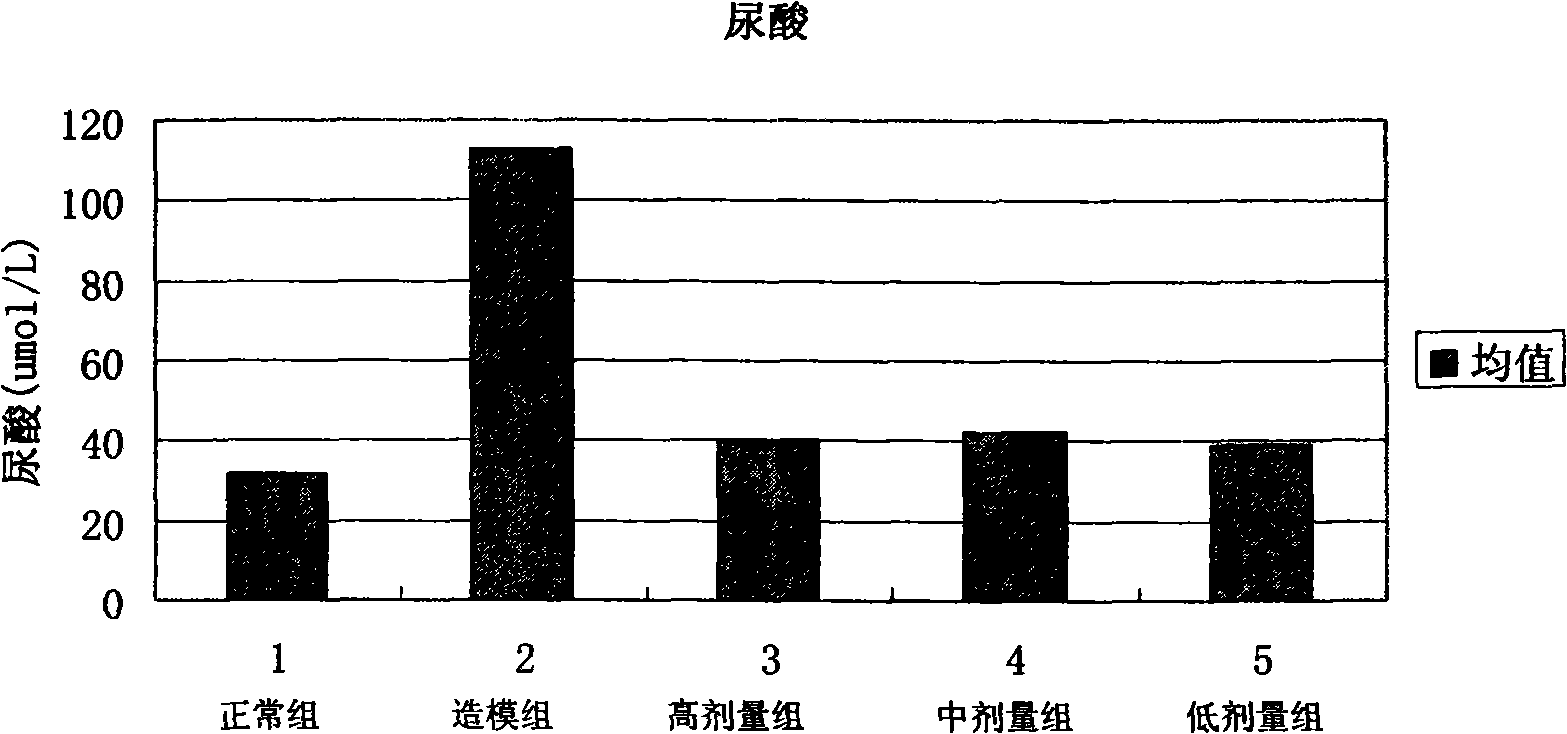
[0060] Clinical application method: for various kidney diseases, the dose of 300-400 μg recombinant human rhBMP-7 / kg·body weight is administered 2-3 times a week for 2-12 consecutive weeks. At the same time, urine output, serum creatinine, uric acid and other indicators were detected.
Embodiment 2
[0062] Measure each component according to the following prescription:
[0063] Bone Morphogenetic Protein-7 200mg
[0064] Urea 60g
[0065] L-Arginine 50g
[0066] Serum Albumin 40g
[0067] Dissolve the above components with water for injection, then set the volume to 1000 ml (pH7.0), so that the final concentration of BMP-7 is 0.2 mg / ml (200 μg / ml); Freeze-dry to prepare the freeze-dried powder injection of bone morphogenetic protein-7.
[0068] The bone morphogenetic protein-7 mentioned above refers to recombinant human rhBMP-7 expressed by mammalian cell COS with a purity of more than 95%, and its amino acid sequence is shown in SEQ ID NO.1.
[0069] Kidney disease application method:
[0070] The powder injection is dissolved in distilled water or saline when used. With the dose of 300-400 μg recombinant human rhBMP-7 / kg body weight, 2-3 times a week. Continuous administration for 2-12 weeks. At the same time, urine output, serum creatinine, uric acid and other ...
Embodiment 3
[0072] Get 300 mg of recombinant human rhBMP-7 expressed by purified prokaryotic cells (purity reaches more than 97%) and add it to 0.3M / L arginine solution (pH6.8-7.4), and use the same concentration of arginine solution to dilute to 1000ml, so that the final concentration of BMP-7 is 0.3mg / ml (300μg / ml), and then after depyrogenation and filter sterilization, it can be divided into 50μg / bottle; 300μg / bottle; 1mg / bottle; 3mg / bottle ; 5mg / bottle; 10mg / bottle injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com