Quality control method for medicament composition for treating knubble
A quality control method and composition technology, which is applied in the direction of drug combination, antineoplastic drugs, and pharmaceutical formulations, and can solve problems that do not involve the determination of astragalus content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0190] Embodiment 1: the preparation of injection of the present invention
[0191] Weigh 250g of Astragalus membranaceus, 120g of ginseng, 8g of matrine, ginseng with 75% ethanol, reflux extraction 3 times, 2 hours each time, combine the extracts, concentrate under reduced pressure to a clear paste with a relative density of 1.10-1.20 , the measured temperature is 65°C, and it is set aside; Astragalus is decocted twice with water, each time for 2 hours, filtered, combined with the filtrate, concentrated under reduced pressure to a clear paste with a relative density of 1.10-1.20, the measured temperature is 65°C, mixed with ginseng clear Combine the pastes, add ethanol to make the alcohol content reach 75%, adjust the pH value to 6-7 with sodium hydroxide, let it stand for 12 hours, take the supernatant, recover the ethanol, concentrate under reduced pressure to a clear liquid with a relative density of 1.10-1.15 Paste, measuring temperature is 65°C; add ethanol to make the a...
Embodiment 2
[0192] Embodiment 2: the preparation of the injection intermediate of the present invention
[0193] Take by weighing 350 parts by weight of Astragalus membranaceus, 70 parts by weight of ginseng, 12 parts by weight of matrine, three flavor raw materials, 75% ethanol for ginseng, reflux extraction 3 times, each 2 hours, combine the extracts, concentrate under reduced pressure to a relative density of 1.10-1.20 clear ointment, measuring temperature is 65 ℃, set aside; Astragalus is decocted twice with water, 2 hours each time, filtered, combined filtrate, concentrated under reduced pressure to clear ointment with relative density of 1.10-1.20, measuring temperature is Combine with ginseng clear paste at 65°C, add ethanol to make the alcohol content reach 75%, adjust the pH value to 6-7 with sodium hydroxide, let stand for 12 hours, take the supernatant, recover ethanol, concentrate under reduced pressure to relative density It is a clear ointment of 1.10-1.15, and the measureme...
Embodiment 3
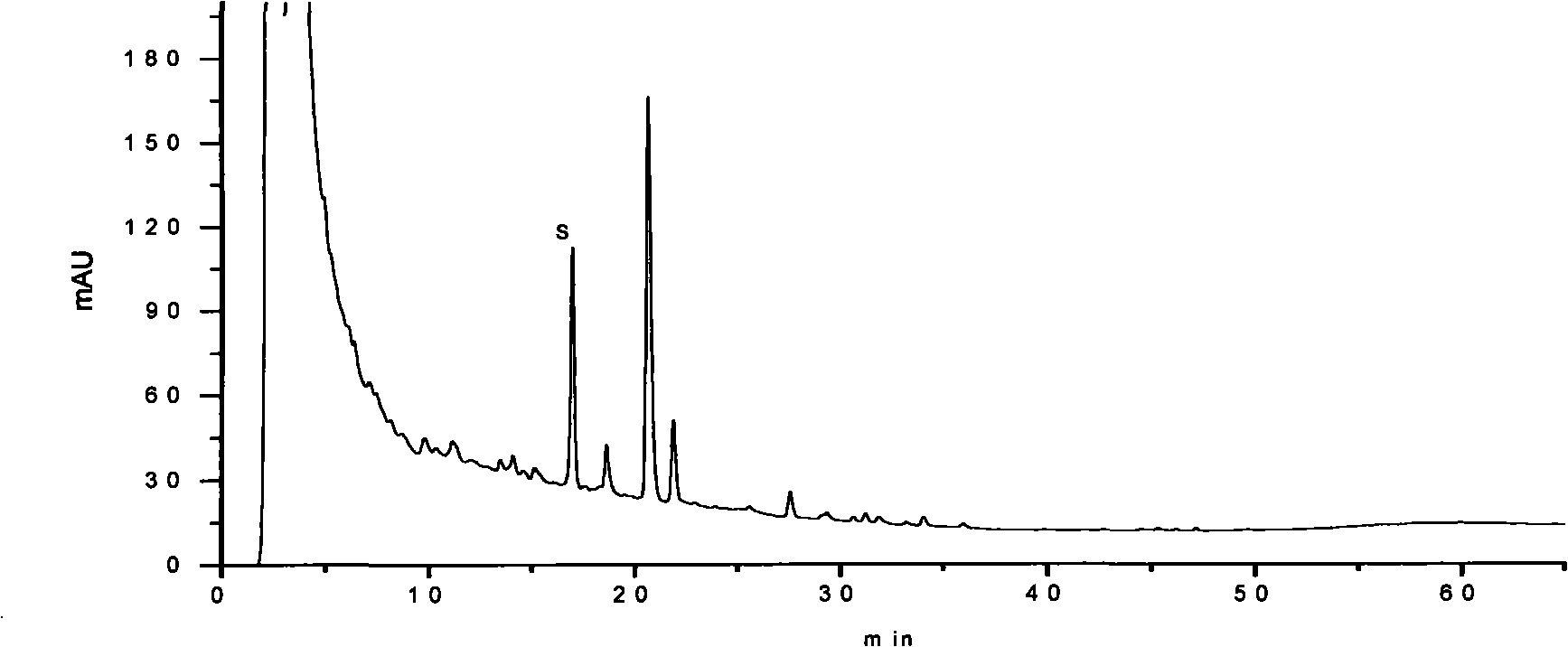
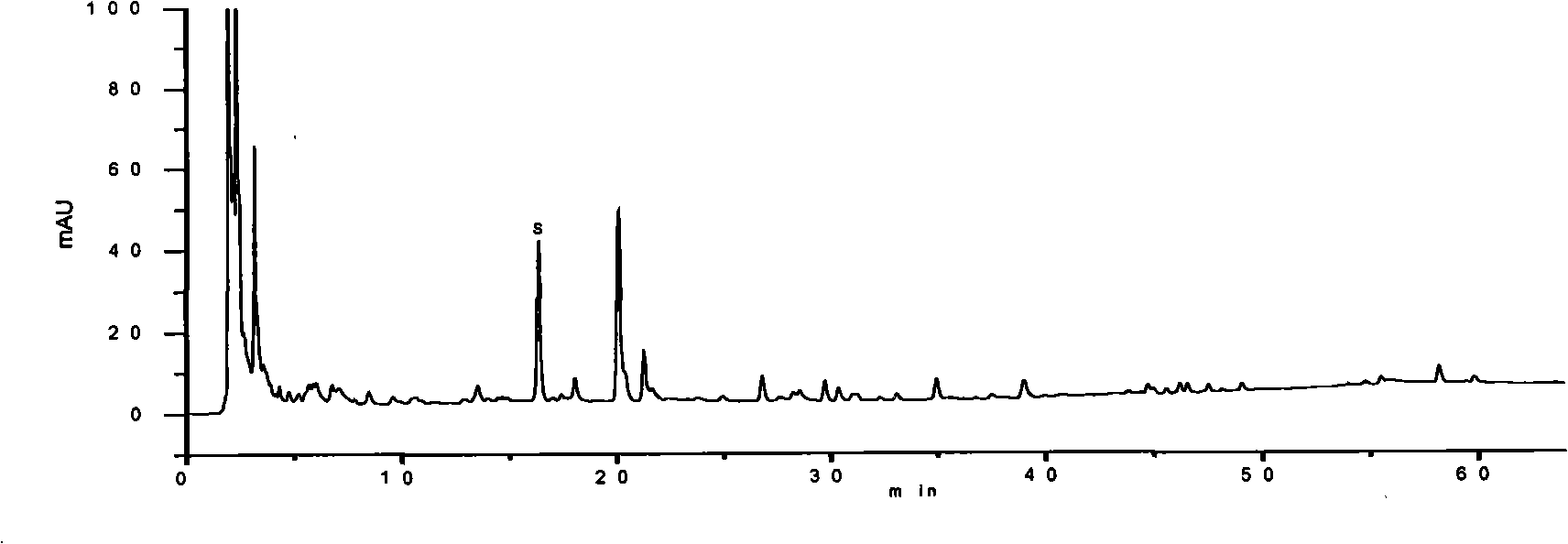
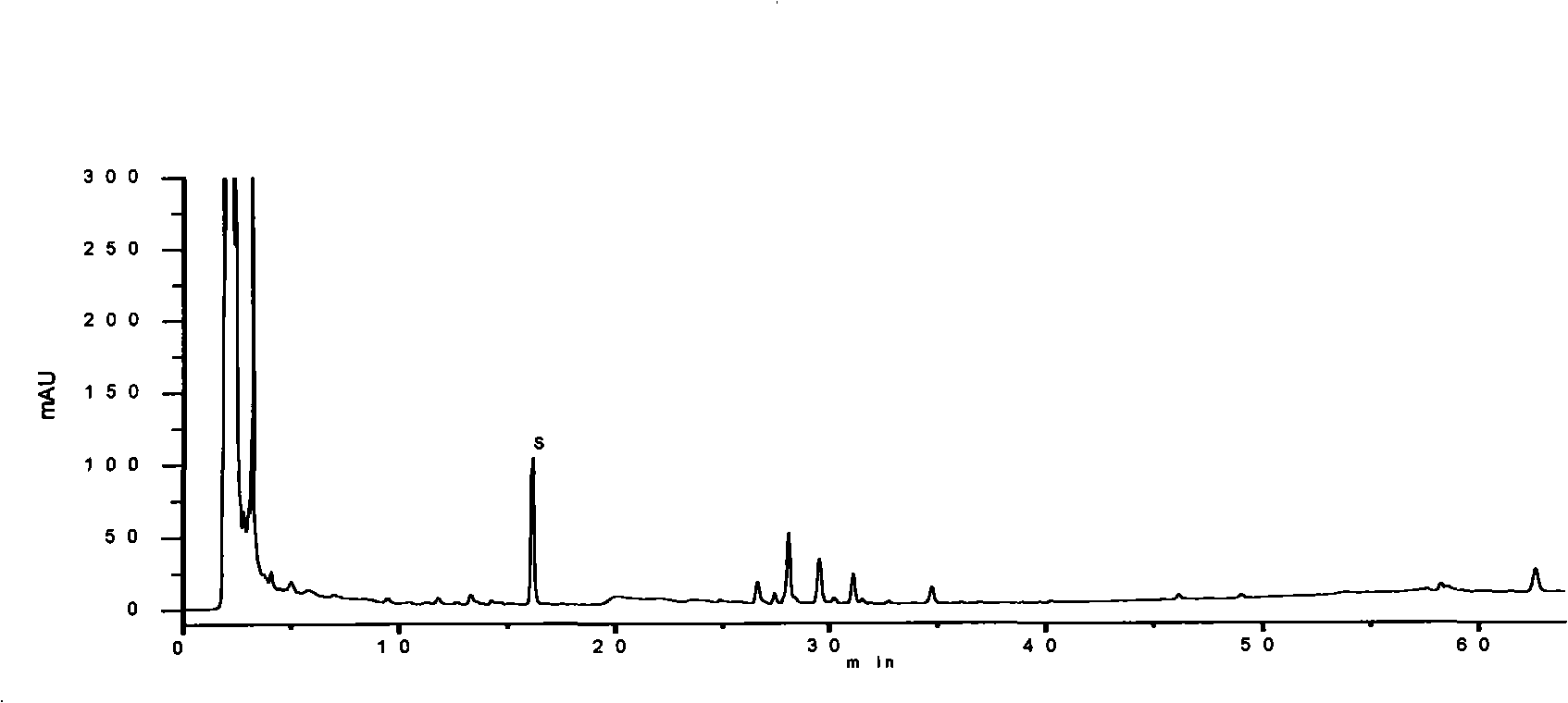
[0194] Embodiment 3: pharmaceutical composition preparation quality control method of the present invention
[0195] Identification:
[0196] Get 20 parts by volume of the pharmaceutical composition preparation of the present invention, evaporate to dryness on a water bath, add 5 parts by volume of methanol to the residue to dissolve, and use it as the test solution; take 1 part by weight of the ginseng reference medicinal material, add 40 parts by volume of chloroform, and heat Reflux for 1 hour, discard the chloroform solution, evaporate the solvent from the dregs, add 0.5 parts by volume of water to stir and moisten, add 10 parts by volume of water-saturated n-butanol, ultrasonicate for 30 minutes, absorb the supernatant and add 3 times the amount of ammonia test solution, Shake well, place to separate layers, take the supernatant and evaporate to dryness, add 1 volume part of methanol to the residue to dissolve, and use it as a reference medicinal solution; then take ginse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com