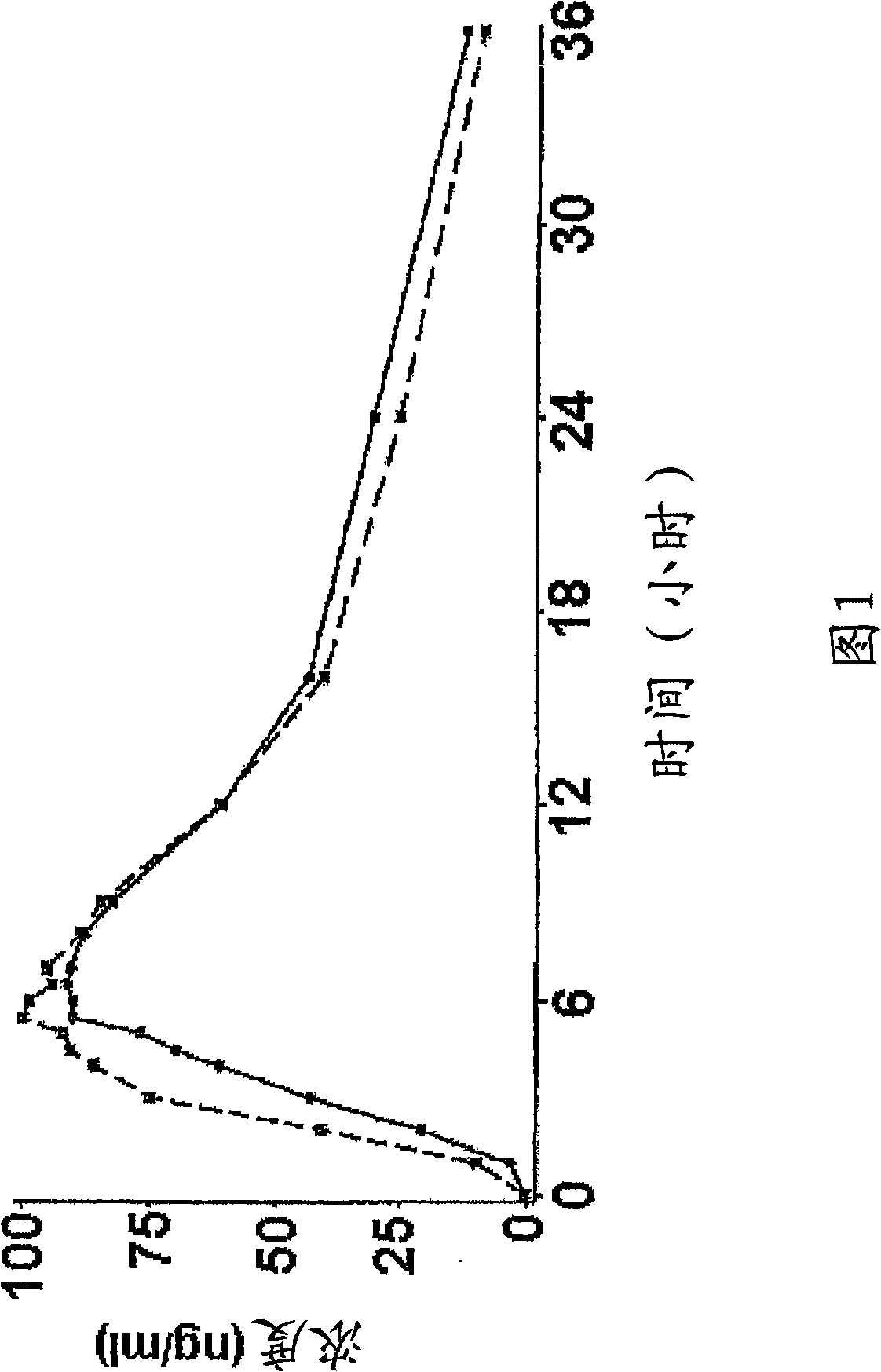
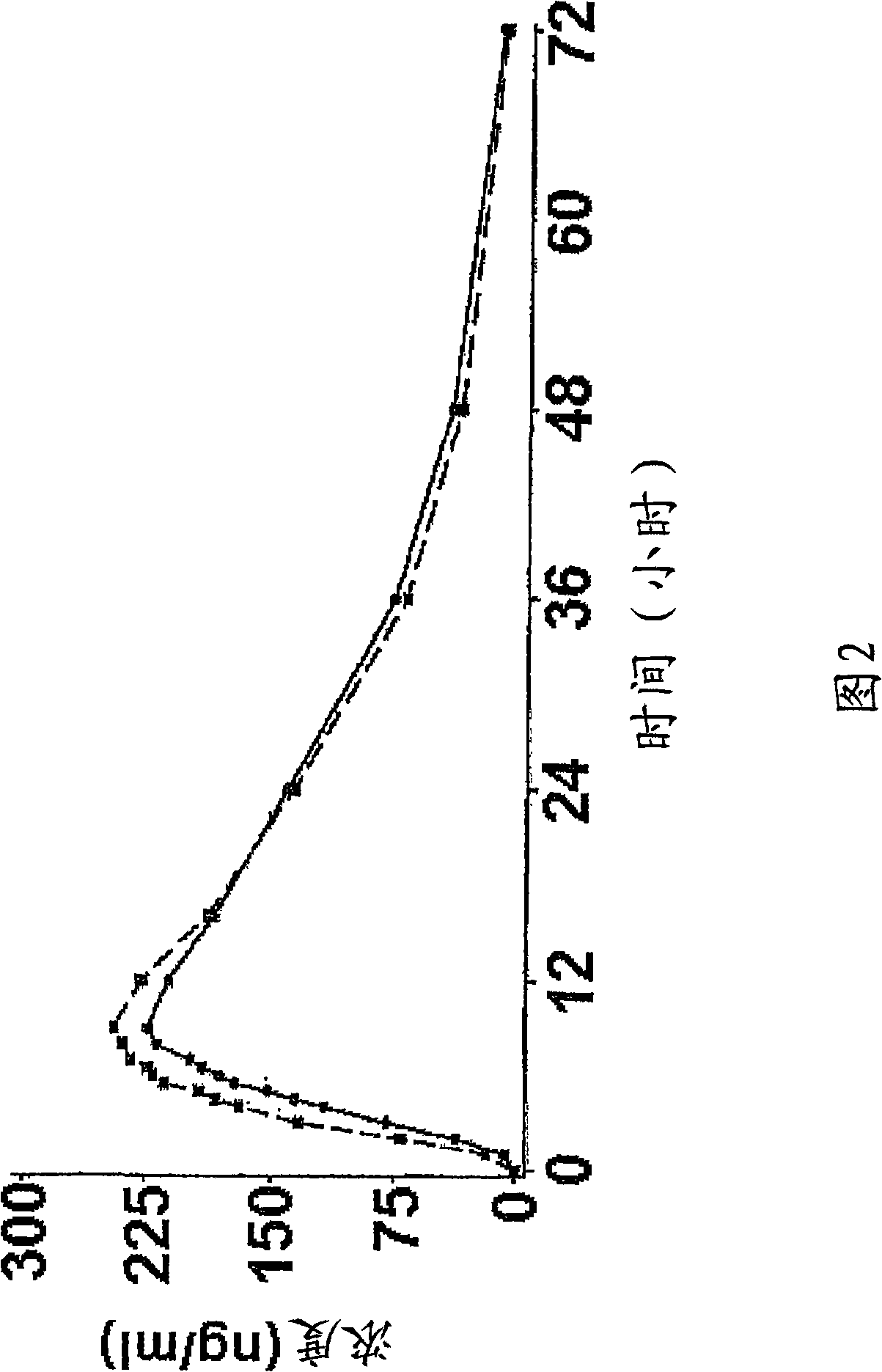
Controlled release pharmaceutical composition of venlafaxine hydrochloride, and process for preparation thereof
A technology of venlafaxine hydrochloride and controlled-release drug, which is applied in the field of novel compositions for controlled-release of venlafaxine hydrochloride, can solve problems such as large-scale preparation of unsuitable pharmaceutical compositions, and achieve good batch-to-batch repeatability. performance, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of active ingredient core
[0030] 10Kg of neutral sugar pellets (composition: 80% by weight of sucrose, 20% of starch) with a particle size distribution of 710-1000 μm were loaded into HP / M025 automatic system GS.
[0031] These neutral pellets were coated with a solution of venlafaxine hydrochloride, which was prepared by dissolving 10 Kg of the active ingredient in 10 Kg of purified water.
[0032] Table 1 below records the operating conditions of the coating and the main operating parameter values set for the equipment.
[0033] Table 1
[0034] Parameter
[0035] The pellets thus obtained were sieved using a 1200 μm mesh and dried at 60°C for 12 hours.
Embodiment 2
[0037] Preparation of controlled release coating
[0038] 290 g of ethyl cellulose and 29 g of stearic acid were added to 2.7 Kg of acetone and 2.7 Kg of 96% ethanol, and the mixture was left to completely dissolve under stirring.
[0039] The solution thus obtained was used to coat the retardation film on the pellets prepared as described in Example 1 above.
[0040] Talc was added in a small amount during the spraying of the solution, and the total amount of talc added was 40 g.
[0041] Table 2 below records the operating conditions of coating delay coating and the main operating parameter values set for the equipment.
[0042] Table 2
[0043] Parameter
[0044] The pellets thus obtained were sieved using a 1340 μm mesh and dried at 60°C for 12 hours. At the end of the drying, the pellets were packed into "0" capsules, which included 150 mg of venlafaxine corresponding to 169.7 mg of venlafaxine hydrochloride.
Embodiment 3
[0046] Preparation of active ingredient core
[0047] The same type of neutral pellets of 2kg sugar spheres as described in Example 1 above were loaded into a GPCG 3 type Glatt fluidized bed, and 50% venlafaxine hydrochloride solution was coated on them.
[0048] At the end of coating the 4Kg solution, the pellets were sieved using a 1200 μm mesh and dried in the same fluidized bed at 60°C for 30 minutes.
[0049] Table 3 below records the operating conditions of the coating and the main operating parameter values set for the equipment.
[0050] table 3
[0051] Parameter
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com