Method for preparing zinc oxide nano hollow spheres by caustic corrosion reaction
A technology of zinc oxide nano and hollow spheres, which is applied in the field of nanomaterials, can solve problems such as uneven size of zinc oxide hollow spheres, blank nano-hollow spheres, complex washing process, etc., and achieve large size controllability and shape controllability, The effect of stable shape and simple process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
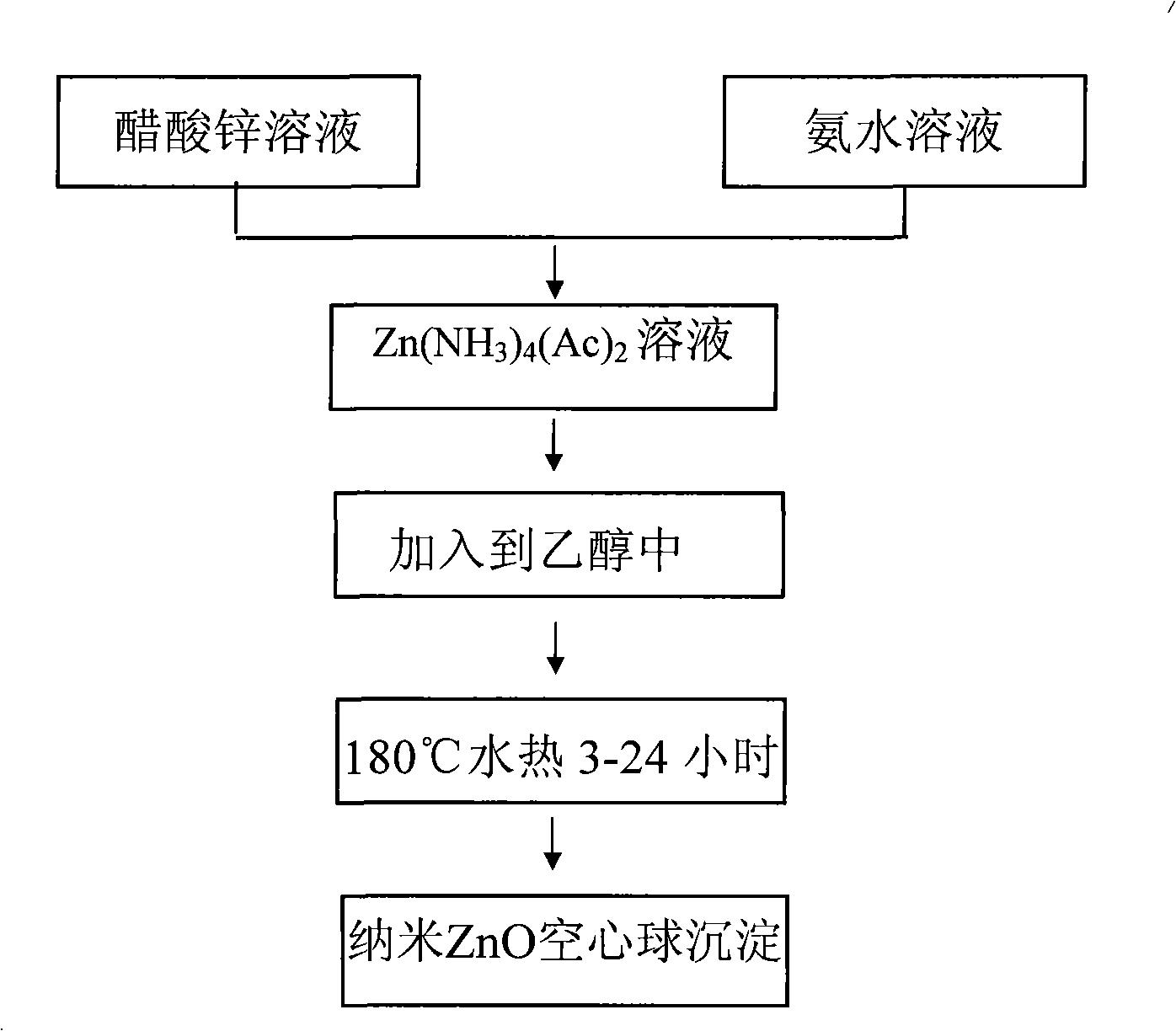
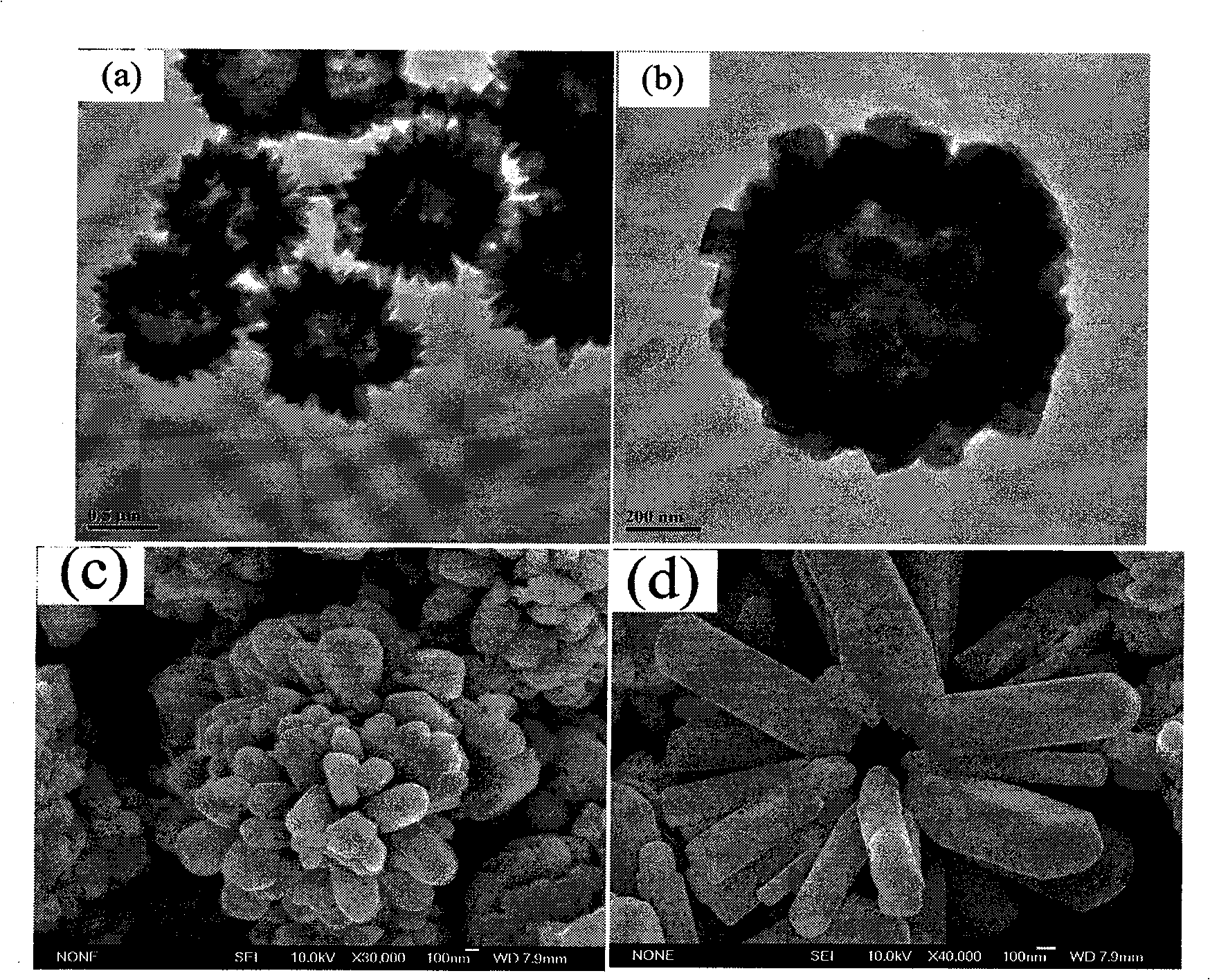
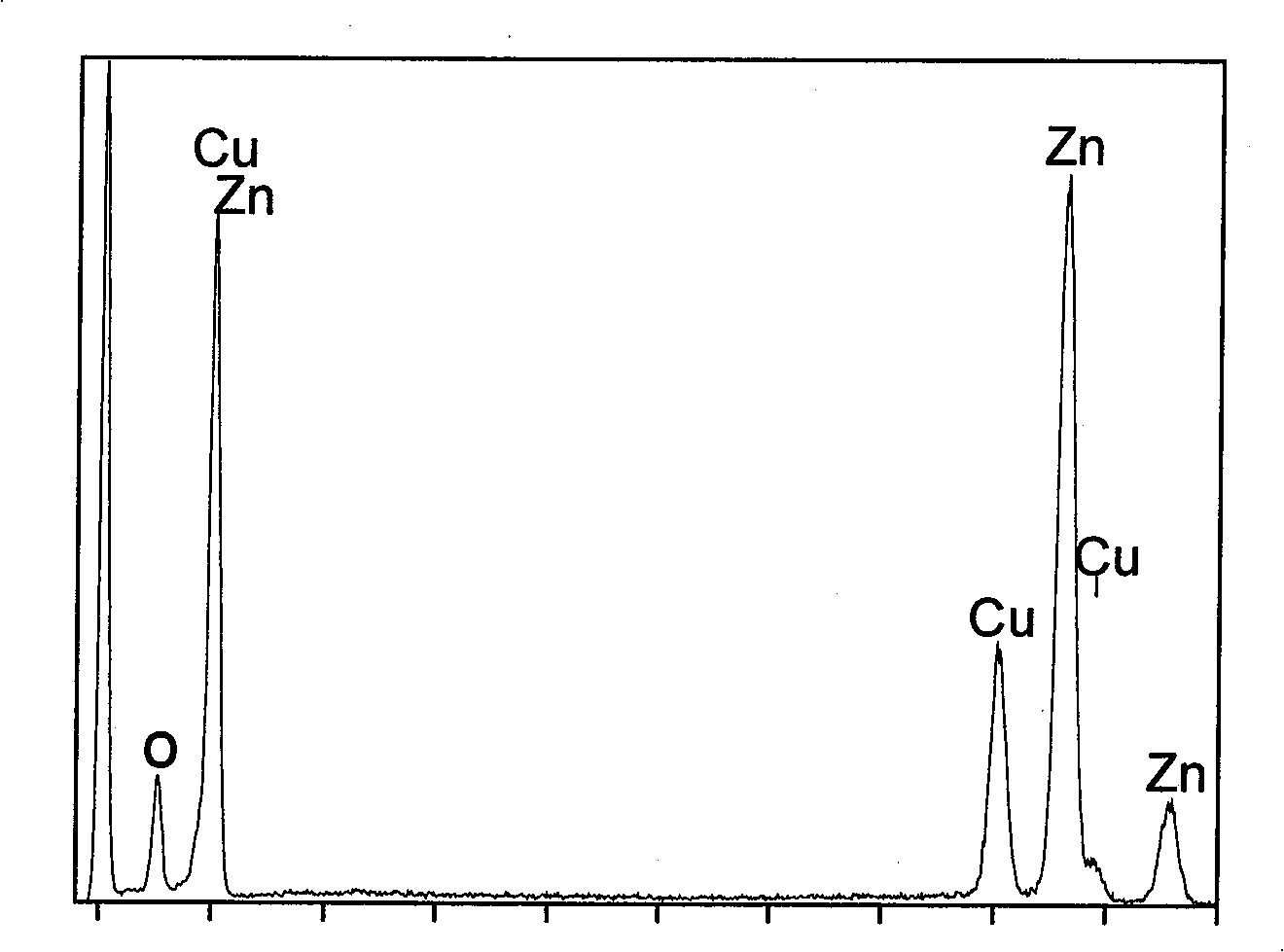
[0032] First prepare solution A: 4.39g Zn(Ac) 2 2H 2 O was dissolved in 100 mL of deionized water and dissolved under stirring at 400-600 rpm. At the same time, solution B: 5M concentrated ammonia solution was prepared. Zn(Ac) in A solution 2 After stable dissolution and complete transparency, add solution B dropwise under magnetic stirring at 400-600 rpm until PH=11.5-14. Due to the addition of ammonia water, solution A immediately produces a white precipitate. With the gradual addition of ammonia water under magnetic stirring, the white precipitate dissolves rapidly and forms a stable Zn(NH 3 ) 4 2+ of ammonia solution. The resulting Zn(NH 3 ) 4 2+ 10 mL of ammonia solution was added to 60 mL of absolute ethanol, then transferred to a 100 mL Teflon tube, and reacted at 180°C for 12 hours to obtain white zinc oxide nanosphere precipitates. Repeat washing with deionized water and absolute ethanol three times each, and finally vacuum-dry at 60° C. for 2 hours. figur...
Embodiment 2
[0034] First prepare solution A: 4.39g Zn(Ac) 2 2H 2 O was dissolved in 100 mL of deionized water and dissolved under stirring at 400-600 rpm. At the same time, solution B: 5M concentrated ammonia solution was prepared. Zn(Ac) in A solution 2 After stable dissolution and complete transparency, add solution B dropwise under magnetic stirring at 400-600 rpm. Due to the addition of ammonia water, solution A immediately produces white precipitate. With the gradual addition of ammonia water under magnetic stirring, the white precipitate dissolves rapidly. Formation of stable Zn(NH 3 ) 4 2+ of ammonia solution. Continue to add solution B dropwise until pH=11.4 to get Zn(NH 3 ) 4 2+ 10 mL of the solution was added to 60 mL of absolute ethanol, transferred to a 100 mL Teflon tube, and reacted at 180°C for 12 hours to obtain a white precipitate. Repeat washing with deionized water and absolute ethanol three times each, and finally vacuum-dry at 60° C. for 2 hours. Figure 5 ...
Embodiment 3
[0036] First prepare solution A: 1.76g Zn(Ac) 2 2H 2 O was dissolved in 100 mL of deionized water and dissolved under stirring at 400-600 rpm. At the same time, solution B: 5M concentrated ammonia solution was prepared. Zn(Ac) in A solution 2 After stable dissolution and complete transparency, add solution B dropwise under magnetic stirring at 400-600 rpm. Due to the addition of ammonia water, solution A immediately produces white precipitate. Under magnetic stirring, with the gradual addition of ammonia water, the white precipitate dissolves rapidly, forming a stable Zn(NH 3 ) 4 2+ The ammonia solution is Zn(NH 3 ) 4 2+ (Ac) 2 . Continue to add solution B dropwise until pH=11.4 to get Zn(NH 3 ) 4 2+ 10 mL of the solution was added to 60 mL of absolute ethanol, transferred to a 100 mL Teflon tube, and reacted at 180°C for 12 hours to obtain a white precipitate. Repeated washing with deionized water 3 times, then washing with absolute ethanol 3 times each, and fin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com