Glonoin Orally disintegrating tablets preparation of and preparing method thereof
A technology of nitroglycerin and disintegrating tablets, which can be used in medical preparations of non-active ingredients, pill delivery, and pharmaceutical formulations, etc. It can solve problems such as poor quality, difficulty in swallowing by patients, and low bioavailability, and achieve rapid disintegration and dissolution rate, relieve the symptoms of angina pectoris, and improve the effect of drug stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Prescription 1:
[0045] The main drug is nitroglycerin solution, and its main drug content is determined according to the weight of the mixture of lactose and xylitol as the auxiliary material in the prescription. The content of the main drug is 0.85ml per 10g of the mixture of lactose and xylitol;
[0046] Excipients and their component weight ratios (excipients and their components weight in g, PVP 30 Alcohol solution in ml)
[0047] Microcrystalline Cellulose 101 40
[0048] Low-substituted hydroxypropyl cellulose 10
[0049] Crospovidone (disintegrant) 6
[0050] Lactose 33
[0051] Xylitol 10
[0052] pvp 30 Alcoholic solution 7.5
[0054] The main ingredient of this embodiment is glycerol absolute ethanol solution (content 10%), binder PVP 30 Alcoholic solution, is 3g PVP 30 Dissolve in 100ml of 50% ethanol as a binder. Cross-linked povidone is selected as the disintegrant; the ratio of microcrystalline cellulose 101 to lo...
Embodiment 2
[0060] Embodiment 2: the preferred polyvinylpolypyrrolidone of present embodiment is as disintegrating agent, is based on following experiment:
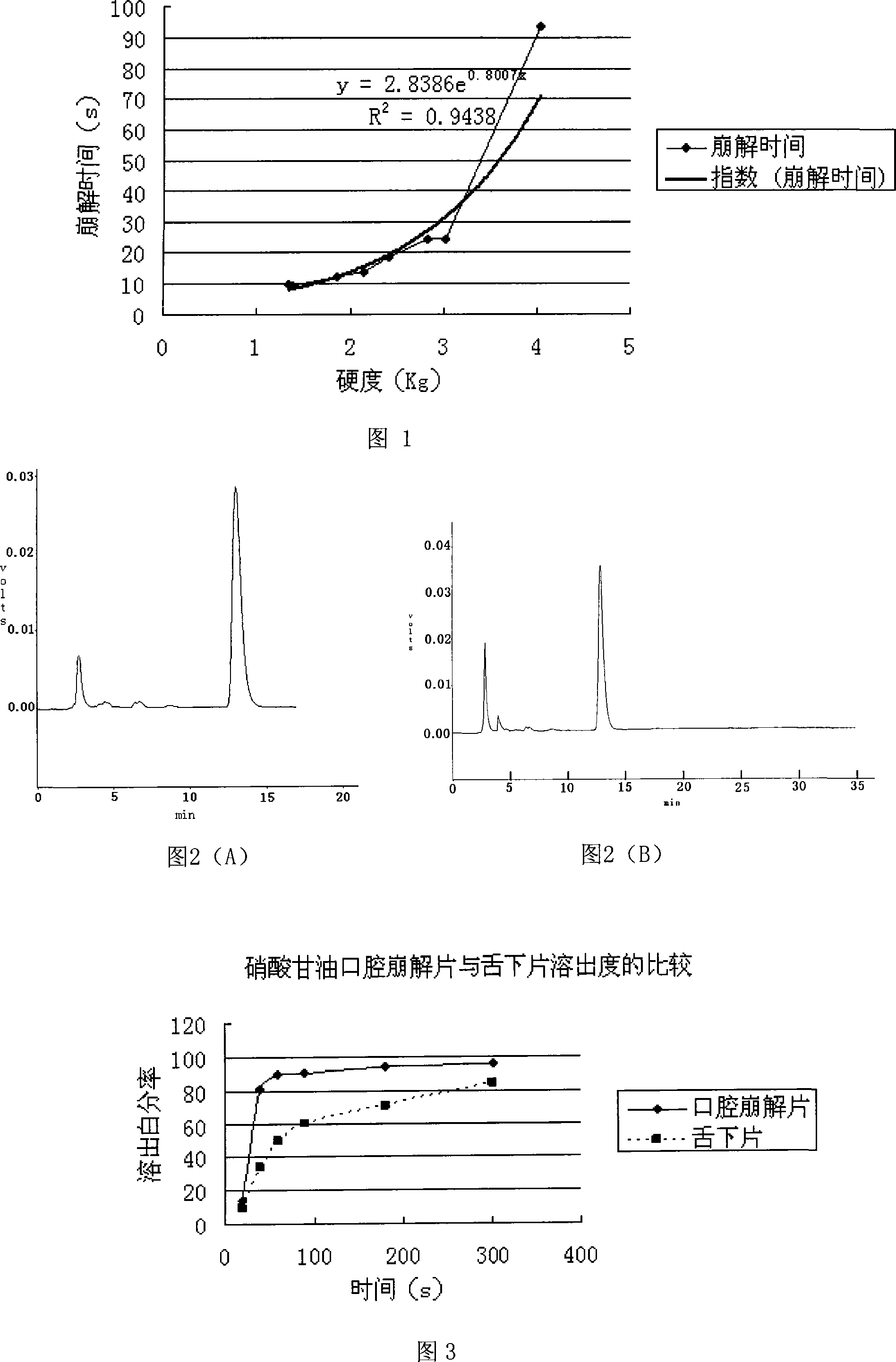
[0061] The coordination of the main drug and the auxiliary materials in the prescription 1 of the above-mentioned embodiment 1 is constant, and the cross-linked povidone, sodium carboxymethyl starch and cross-linked carboxymethyl Sodium cellulose was used as a disintegrant respectively, and three kinds of disintegrating tablets were prepared according to the preparation process of the above-mentioned Example 1, and the disintegration time of the obtained tablets was measured. Table 1 is the experimental data of disintegration time, and Table 2 is the result of mathematical statistics.
[0062] The experimental data of table 1 disintegration time
[0063] group
[0064] Table 2 One-way analysis of variance (disintegration time)
[0065] sum of squares
[0066] The results of variance analysis showed that the di...
Embodiment 3
[0068] According to embodiment 1 prescription 1, the coordination of main ingredient and auxiliary material remains unchanged, and the binder in the auxiliary material prepared by it by weight ratio is made into 3% PVP respectively with 75% ethanol, 50% ethanol, and 0% ethanol 30 Alcoholic solution and 3% PVP 30 Aqueous solution, respectively prepare three kinds of orally disintegrating tablets according to the preparation process of the above-mentioned Example 1. The disintegration time (s) is measured, and Table 3 is the experimental data obtained. Table 4 is the result of mathematical statistics.
[0069] Table 3 Experimental data
[0070] group
[0071] Table 4 One-way analysis of variance (disintegration time)
[0072] sum of squares
[0073] Between groups
[0074] The results of variance analysis showed that the disintegration time of the orally disintegrating tablets made with three different binders was statistically different. Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com