Aminoglycoside derivatives
A technology of aminoglycosides and drugs, applied in the field of medicine, can solve problems such as severe ototoxicity and nephrotoxicity, and easy drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
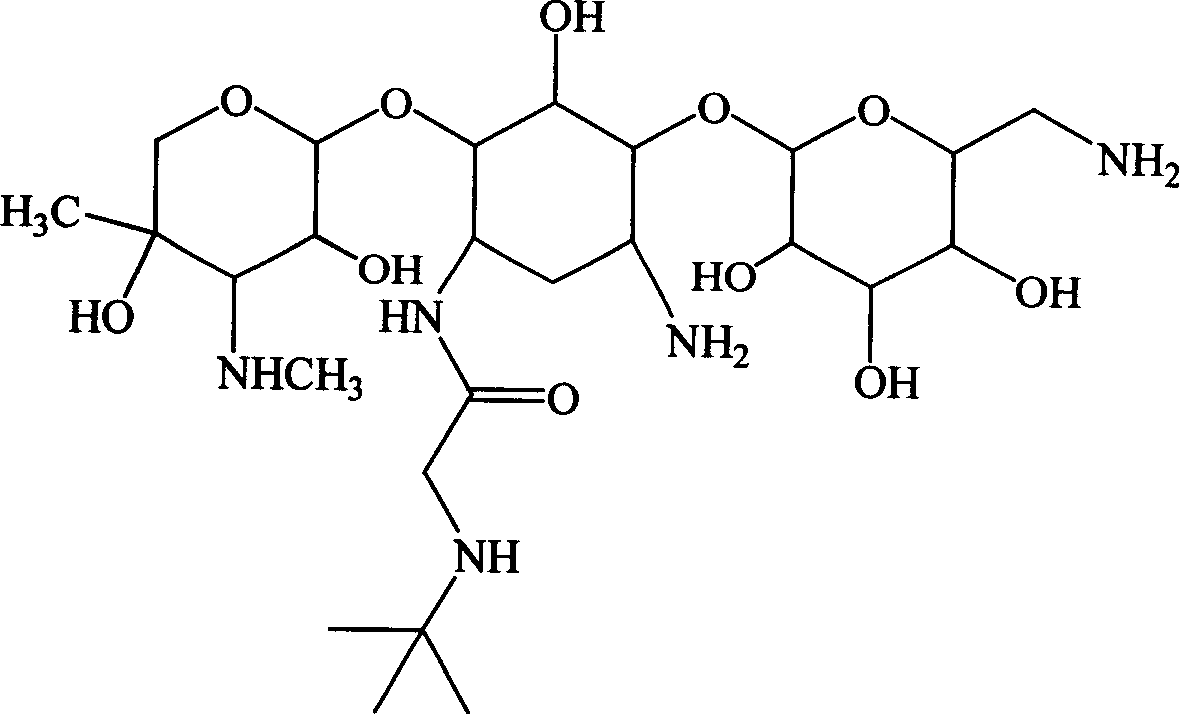
[0112] Preparation of embodiment 11-N-tert-butylaminoacetyl-gentamycin B and sulfate thereof
[0113] Step 1: Preparation of tert-butylaminoacetic acid active ester
[0114]
[0115] In a dry there-necked flask, add 100ml of anhydrous acetonitrile, add 4g (30.5mmol) of tert-butylaminoacetic acid under stirring, after stirring and dissolving, add 4.8g (35.5mmol) of 1-hydroxybenzotriazole (HOBT), and use The temperature in the water bath was raised to 40°C, stirred and reacted for 1 hour, then lowered to room temperature, 7.4 g (36 mmol) of dicyclohexylcarboimide was added in batches, stirred and reacted at room temperature for 2 hours, the reaction solution was lowered to 0°C, and after standing Filter and use the filtrate for later use.
[0116] Step 2: Preparation of silylated gentamicin B
[0117] Add 30ml of acetonitrile and 60ml of ethylene glycol dimethyl ether into a dry three-necked reaction flask equipped with a reflux condenser with a drying tube and a stirring...
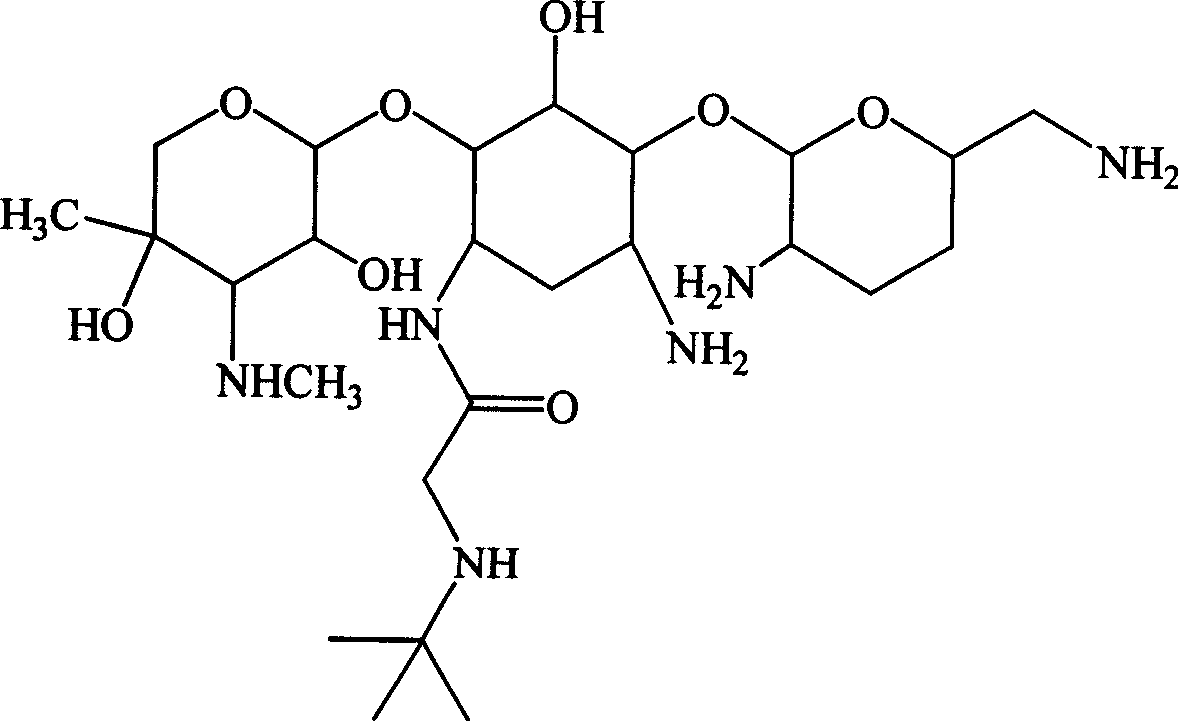
Embodiment 2
[0126] Example 2 1-N-tert-butylaminoacetyl-gentamycin C 1a and its sulfate preparation
[0127] Referring to Example 1, feeding gentamicin C 1a 11.2g (25mmol), to get 1-N-tert-butylaminoacetyl-gentamycin C 1a Sulfate 11.2g, total yield: 59.1%.
[0128] Molecular formula: C 25 h 50 N 6 o 8 2H 2 SO 4
[0129] Molecular weight: 758.86
[0130] Elemental Analysis: Found: C, 39.48%; H, 7.30%; N, 11.15%; S, 8.41%
[0131] Theoretical value: C, 39.57%; H, 7.17%; N, 11.07%; S, 8.45%
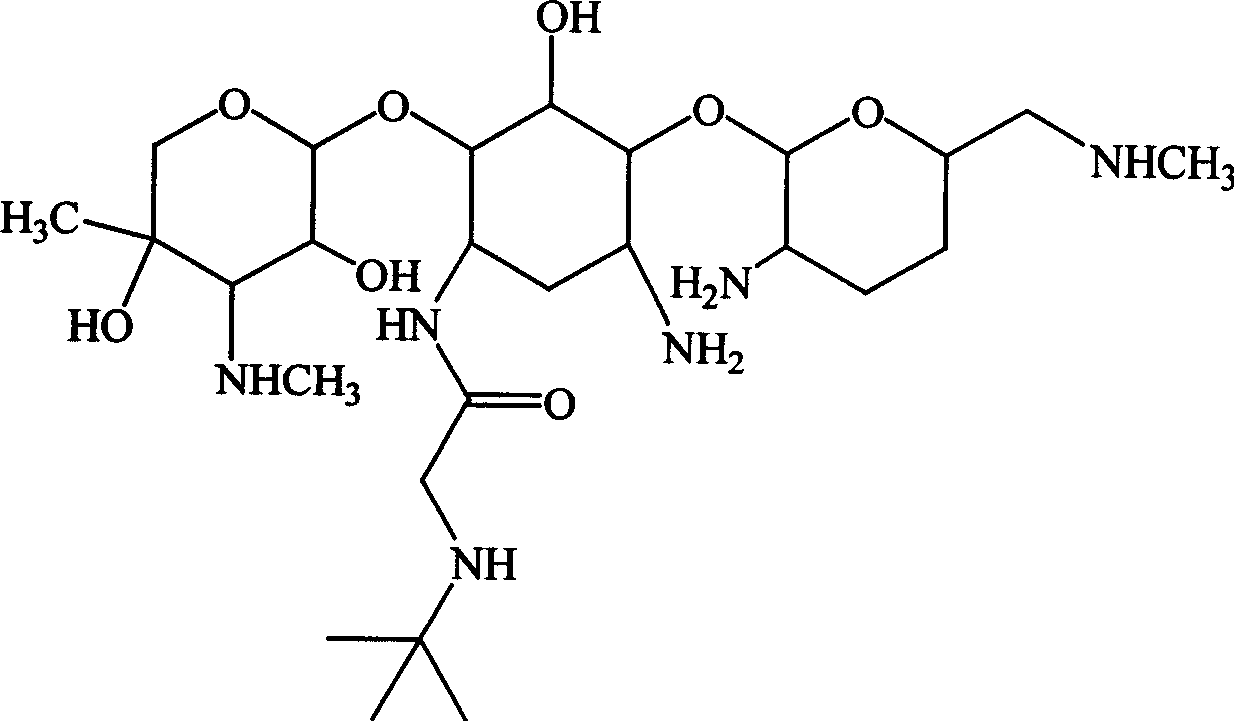
Embodiment 3
[0132] Example 3 1-N-tert-butylaminoacetyl-gentamycin C 2b preparation of
[0133] Referring to Example 1, feeding gentamicin C 2b 11.6g (25mmol), to get 1-N-tert-butylaminoacetyl-gentamycin C 2b Sulfate 10.5g, total yield: 54.3%.
[0134] Molecular formula: C 26 h 52 N 6 o 8 2H 2 SO 4
[0135] Molecular weight: 772.88
[0136] Elemental Analysis: Found: C, 40.26%; H, 7.45%; N, 10.79%; S, 8.24%
[0137] Theoretical value: C, 40.40%; H, 7.30%; N, 10.87%; S, 8.30%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com