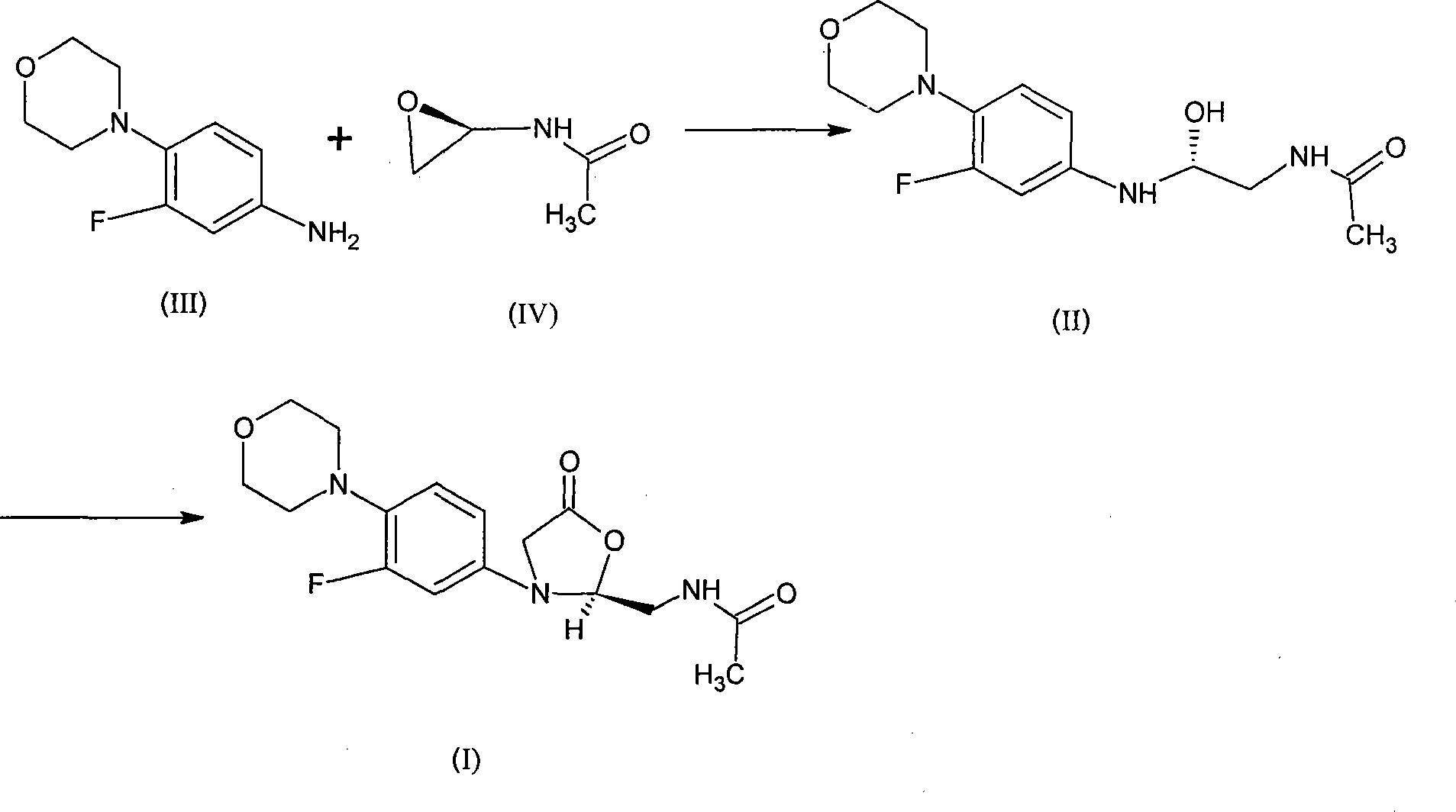
Synthesis of linezolid
A technology of linezolid and its synthesis method, applied in organic chemistry, antibacterial drugs, etc., can solve problems such as danger, long reaction route, unfavorable safety production, etc., and achieve the effect of mild reaction conditions and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Dissolve 34.6mmol (6.8g) 3-fluoro-4-morpholinoaniline and 38.1mmol (3.85g) N-2(R)-oxirane amide in 50ml isopropanol and react at 80℃ for 20 hours After the solvent was distilled off under reduced pressure, the residue was dissolved with a small amount of dichloromethane and separated by silica gel column chromatography to obtain N-{2(R)-2-[(3-fluoro-4morpholin-4-ylphenyl) )Amino]-2-hydroxyethyl}acetamide 5.98g, the yield is 58.2%.
[0029] Dissolve 20.1mmol (5.98g) N-{2(R)-2-[(3-fluoro-4morpholin-4-ylphenyl)amino]-2-hydroxyethyl}acetamide in 30ml dichloromethane After adding 4ml of pyridine, cool to 0℃ with an ice-water mixture, dissolve 7.4mmol (2.2g) triphosgene solid in 20ml of dichloromethane, slowly add dropwise to the above solution, after the addition, stir at 25℃ for reaction After 2 hours, add triethylamine to adjust the pH to weakly alkaline, extract with dichloromethane, wash with water, saturated brine, dry with anhydrous magnesium sulfate, filter, and evaporate...
Embodiment 2
[0031] Dissolve 25.5mmol (5g) 3-fluoro-4-morpholinoaniline and 28.2mmol (2.85g) N-2(R)-oxirane amide in 35ml isopropanol and react at 70°C for 18 hours, After the solvent was distilled off under reduced pressure, it was dissolved in a small amount of dichloromethane, and separated by silica gel column chromatography to obtain N-{2(R)-2-[(3-fluoro-4morpholin-4-ylphenyl)amino] -2-hydroxyethyl}acetamide 4.5g, yield 59.5%
[0032] Dissolve 15.2mmol (4.5g) N-{2(R)-2-[(3-fluoro-4morpholin-4-ylphenyl)amino]-2-hydroxyethyl}acetamide with 25ml dichloromethane After adding 3ml pyridine, cool to 0℃ with ice-water mixture, dissolve 5.1mmol (1.5g) triphosgene solid in 15ml dichloromethane, slowly add dropwise to the above solution, after addition, stir at 20℃ for reaction After 2 hours, add triethylamine to adjust the pH to weakly alkaline, extract with dichloromethane, wash with water, saturated brine, dry with anhydrous magnesium sulfate, filter, and evaporate the solvent under reduced press...
Embodiment 3
[0034] Dissolve 34.6mmol (6.8g) 3-fluoro-4-morpholinoaniline and 38.1mmol (3.85g) N-2(R)-oxirane amide in 50ml tetrahydrofuran. The reaction temperature is 60℃ to obtain N-{ 2(R)-2-[(3-Fluoro-4morpholin-4-ylphenyl)amino]-2-hydroxyethyl}acetamide 5.6 g, yield 54.5%. The other steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com