Rhenium(I) tricarbonyl polypyridyl complex and its preparation method and use
A complex, tricarbonyl chloride technology, applied in the field of dye-sensitized solar cells, to achieve the effects of purification, good stability, and distortion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
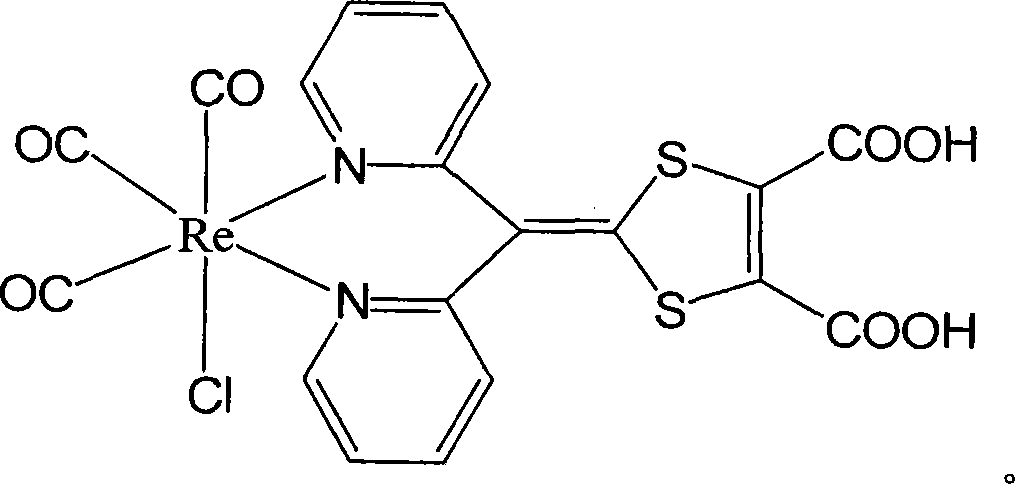
[0024] Example 1: Preparation of tricarbonyl chloride {4-[bis(2-pyridyl)methylene]-3,5-dithiole-1,2-dicarboxylic acid}rhenium(I)
[0025] 1.4-[bis(2-pyridyl)methylene]-3,5-dithiol-1,2-dicarboxylic acid methyl ester (A):
[0026] a. In 3,4-dimethoxycarbonyl-2,5-dithiol-3-enethione (1.00g, 4mmol) and bis(2-pyridine)methanone under nitrogen gas conditions (0.74g, 4mmol) were added 7ml of toluene and 5ml of triethoxyphosphine into the mixture, and refluxed for 6 hours. After stopping the reaction, cool to -20°C, filter, and wash with ether to obtain red crystal 4-[bis(2-pyridyl)methylene]-3,5-dithiole-1,2-dicarboxylic acid methyl Esters 0.83g, yield 53.7%. Compound by 1 H-NMR, elemental analysis, mass spectrometry, and infrared were verified, and the results showed that the structure was correct. The data are as follows:
[0027] 1 H-NMR (500MHz, CDCl 3 ,δ):
[0028] 8.79(d, J=3.6Hz, 2H) 7.72(t, 2H) 7.22(t, 2H) 7.17(d, J=7.9Hz, 2H) 3.87(s, 6H)
[0029] Elemental analysis r...
Embodiment 2
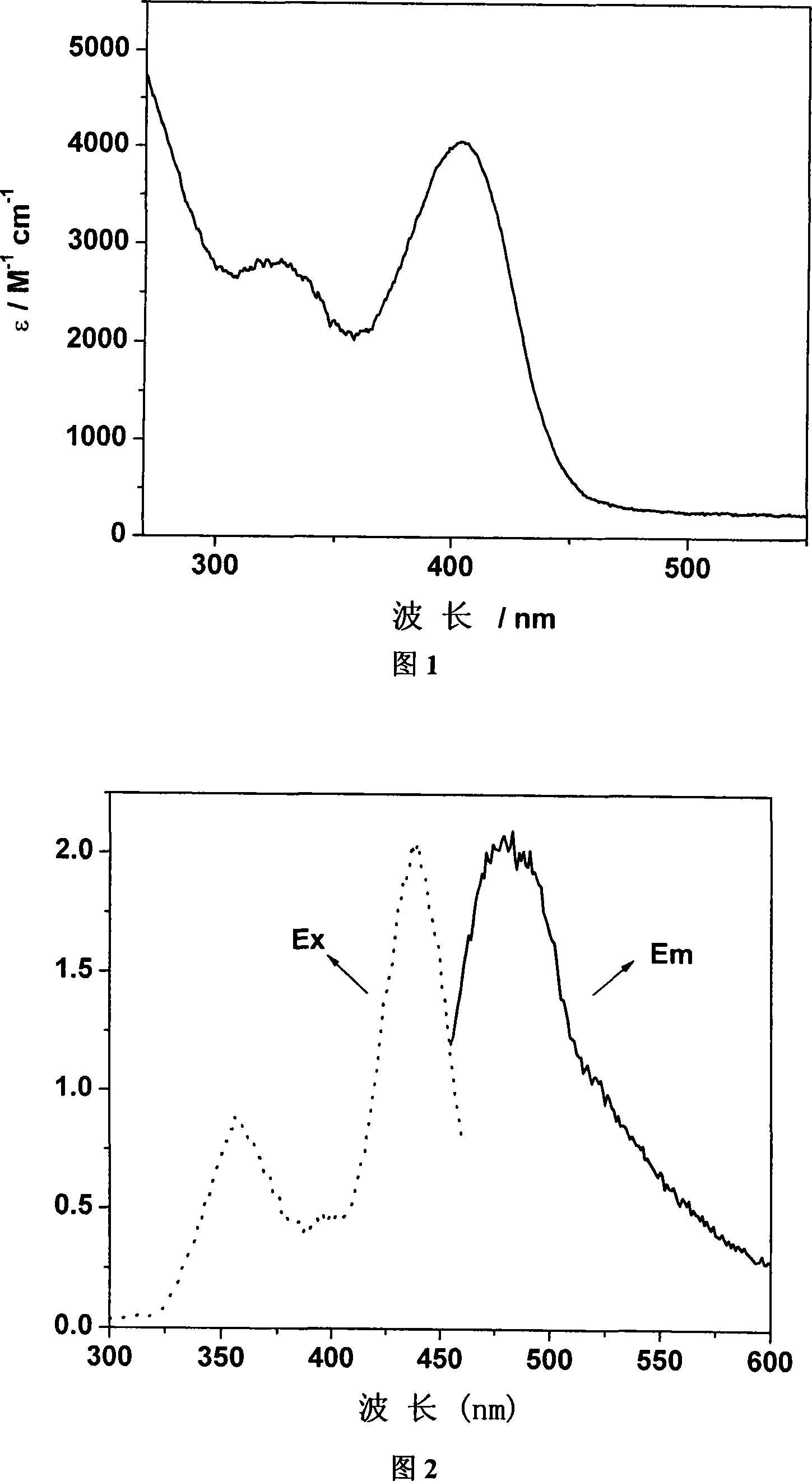
[0053] Example 2: Chlorinated tricarbonyl {4-[bis(2-pyridyl)methylene]-3,5-dithiole-1,2-dicarboxylic acid}rhenium(I) of the present invention Fluorescent characterization of
[0054] The test instrument is AMINCO Bowman Series 2 Fluorometer. Complex C of the present invention is dissolved in methanol and measured (5 × 10 -4 M). At room temperature, the excitation peak λ ex 356nm and 440nm, emission peak λ em It is 483nm (see accompanying drawing 2).
Embodiment 3
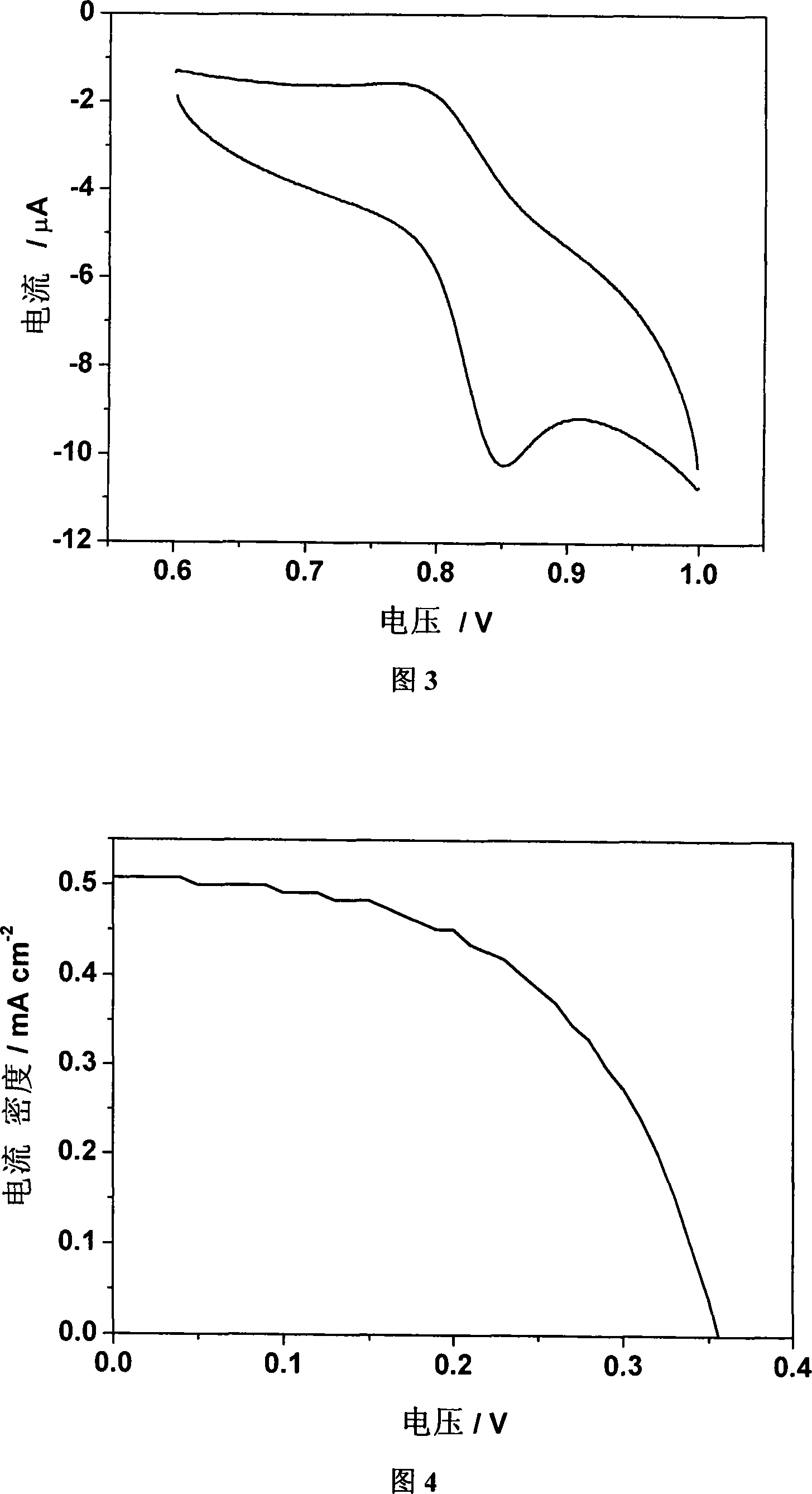
[0055] Example 3: Chlorinated tricarbonyl {4-[bis(2-pyridyl)methylene]-3,5-dithiole-1,2-dicarboxylic acid} rhenium(I) of the present invention Electrochemical characterization of
[0056] The test instrument is CHI660b electrochemical workstation. Glassy carbon electrode, platinum wire electrode, Ag / Ag + They were used as working electrode, counter electrode and reference electrode respectively. Complex C of the present invention was dissolved in DMF (5 × 10 -4 M), with 0.1Mn-Bu 4 NClO 4 For the supporting electrolyte, the scan speed is 100mV s -1 . The test results show that there is a pair of redox peaks, and the half-wave potential value is 0.82V, which is the redox potential of metal rhenium from positive one valence to positive two valence (see Figure 3).
PUM
| Property | Measurement | Unit |
|---|---|---|
| open-circuit voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com