A polyurea product as thixotropic rheology modifying agent
一种触变剂、聚脲的技术,应用在触变涂料、油墨、涂层等方向,能够解决结晶结构不能发挥流变作用、增强光学效应等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The preparation of each of the first and second polyurea compounds can also optionally be carried out in the presence of a binder, and it is of course preferred that the first polyurea compound is prepared as a colloid in the environment of a host resin. This can be done by mixing a mixture of the binder and isocyanate with the amine component or by mixing the isocyanate with a mixture of the binder and amine component, or by mixing the binder separately with the amine This is done by mixing the two mixtures of components and NCO-components. Obviously, if the base material is highly reactive with the amines or isocyanates, the base material and the particularly sensitive compound cannot be premixed. The term "highly reactive" herein means that more than 30% of the sensitive amine or isocyanate reacts with the base material before mixing the amino acid derivative and isocyanate to prepare the rheology modifier. The mixing operation may be carried out in any convenient m...
Embodiment 1
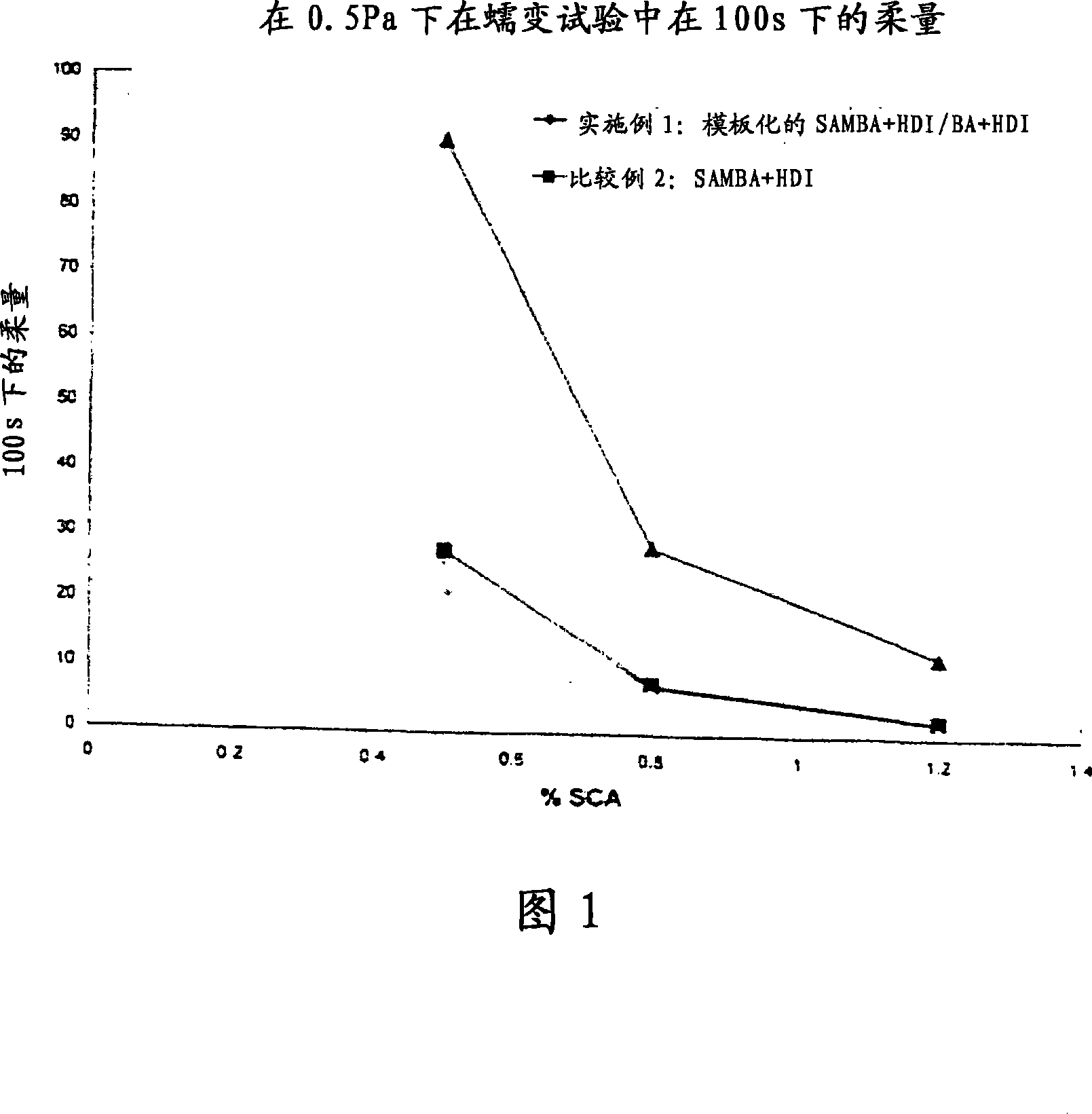
[0101] (90 / 10)S / R-AMBA+HDI / BA+HDI (1:1) in Setal 166 SS-80
[0102] 100.0 g Setal 166 SS-80 was added to the reactor, and it was mixed with 0.06 g (0.5 mmol) R-(+)-α-methylbenzylamine and 0.58 g (4.8 mmol) S- (-)-α-Methylbenzylamine was mixed for 2 minutes. The stirring speed was increased to 4000 rpm, and 0.46 g (2.7 mmol) of 1,6-hexamethylene diisocyanate was added using a syringe. The mixture was stirred for a further 30 seconds at 4000 rpm. The stirring speed was reduced to 1000 rpm and after 2 minutes 0.60 g (5.6 mmol) benzylamine was added. A mixture of 0.49 g (2.91 mmol) of 1,6-hexamethylene diisocyanate and 0.49 g of butyl acetate was added to the reactor within 15 minutes using a pump. The mixture was stirred for an additional 30 seconds at 1000 rpm.
Embodiment 1A
[0104] (90 / 10) S / R-AMBA+HDI / BA+HDI (1:1) in Setal 166 SS-80 100.0 g of Setal 166 SS-80 was added to the reactor and the This was mixed with 0.06 g (0.50 mmol) of R-(+)-α-methylbenzylamine and 0.58 g (4.8 mmol) of S-(-)-α-methylbenzylamine for 2 minutes. The stirring speed was increased to 4000 rpm, and 0.46 g (2.7 mmol) of 1,6-hexamethylene diisocyanate was added using a syringe. The mixture was stirred for a further 30 seconds at 4000 rpm. The stirring speed was reduced to 1000 rpm and after 5 minutes 0.60 g (5.6 mmol) benzylamine was added. The mixture was stirred for a further 2 minutes at 1000 rpm. The stirring speed was increased to 1500 rpm, and 0.50 g (2.97 mmol) of 1,6-hexamethylene diisocyanate was added using a syringe. The mixture was stirred for an additional 30 seconds at 1000 rpm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| shear viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com