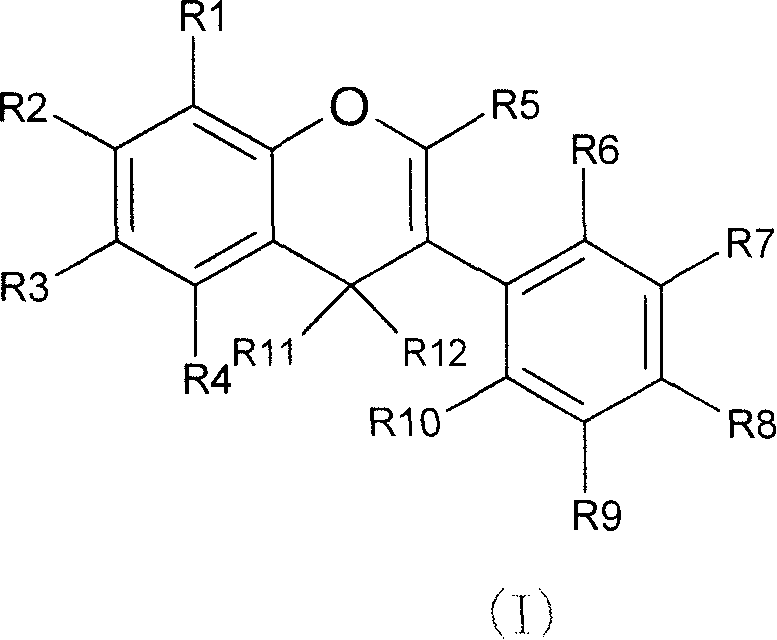
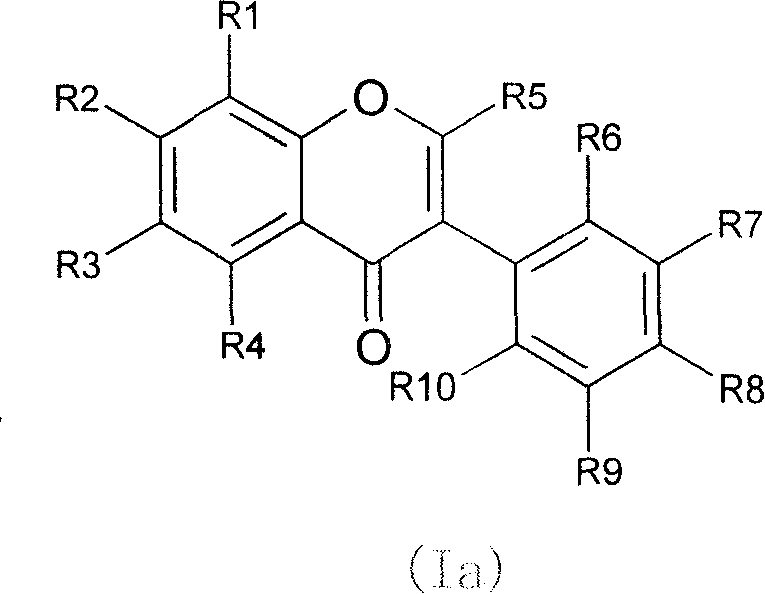
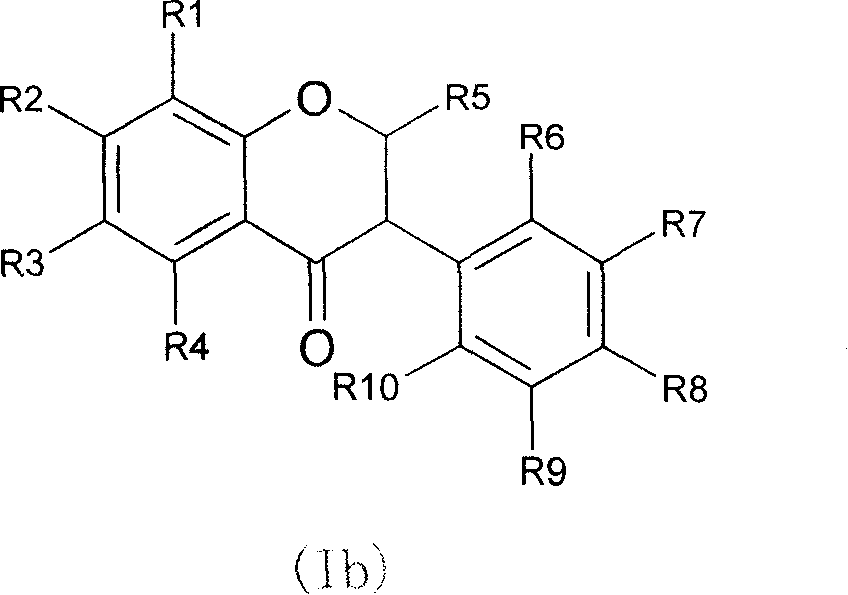
Novel isoflavone nicotinic acid ester derivatives, preparing method and use thereof
A technology of nicotinic acid isoflavone ester and isoflavone ester, which is applied in the field of chemical compounds and can solve problems such as failure to effectively solve problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Preparation of 4',5-di-hydroxy-7-nicotinic acid isoflavone ester (code 101) and 5-hydroxy-4',7-di-nicotinic acid isoflavone ester (code 102):
[0089] The 0.01mol 4′,5,7-trihydroxyisoflavone 1 was reacted with 0.03mol niacin in 10ml thionyl chloride at 72℃ for 9 hours, poured into an ice water bath, and extracted with ethyl acetate 3 times. Drying with sodium sulfate and then column chromatography with ethyl acetate: petroleum ether = 1:3 to obtain the target product 4',5-di-hydroxy-7-nicotinic acid isoflavone ester 2 with a yield of 45.2% and 5-hydroxy- 4',7-Di-nicotinic acid isoflavone ester 3, the yield is 52.6%. The relevant data are as follows:
[0090] 4',5-Di-hydroxy-7-nicotinic acid isoflavone 2 (code 101) MS (EI, 70ev) m / z: 375; 1 H NMR(300MHz, CDCl 3 ): 6.29 (2H, s), 6.98 (2H, d, J = 8.1 Hz), 7.12 (2H, d, J = 8.1 Hz), 7.48 (1H, s), 7.58-9.02 (4H, m), 9.50 (1H, s), 12.04 (1H, s); Anal.Calcd.forC 21 H 13 NO 6 : C, 67.20, H, 3.49, N 3.73; Found C, 67.19, H, 3.50, N 3...
Embodiment 2
[0092] Preparation of 4',5-di-methoxy-7-nicotinic acid isoflavone ester (code number 103):
[0093] Dissolve 0.03mol methyl iodide and 0.01mol 4′,5-di-hydroxy-7-nicotinic acid isoflavone ester 2 in 25ml acetone, add 10g catalyst K 2 CO 3 And react at 60°C for 4 hours to obtain the target product 4',5-di-methoxy-7-nicotinic acid isoflavone ester. The relevant data are as follows:
[0094] MS (EI, 70ev) m / z: 403; Anal.Calcd.for C 23 H 17 NO 6 : C, 68.48, H, 4.25, N3.47; Found C, 68.51, H, 4.21, N3.52.
Embodiment 3
[0096] Preparation of 4',5-di-ethoxy-7-nicotinic acid isoflavone ester (code number 104):
[0097] Using 0.03 mol of bromoethane instead of methyl iodide, the compound 4',5-di-ethoxy-7-nicotinic acid isoflavone ester can be obtained according to the operation of Example 2. The relevant data are as follows:
[0098] MS (EI, 70ev) m / z: 43 1; Anal.Calcd.for C 25 H21 NO 6 : C, 69.60, H, 4.91, N3.25; Found C, 69.33, H, 4.81, N3.22.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com