Process for preparing cerebroprotein hydrolysate NaCl injection
A technology of cerebroprotein hydrolyzate and sodium chloride injection, which is applied in the field of preparation of cerebroprotein hydrolyzate sodium chloride injection, can solve the problems of reduced yield, danger, and low production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
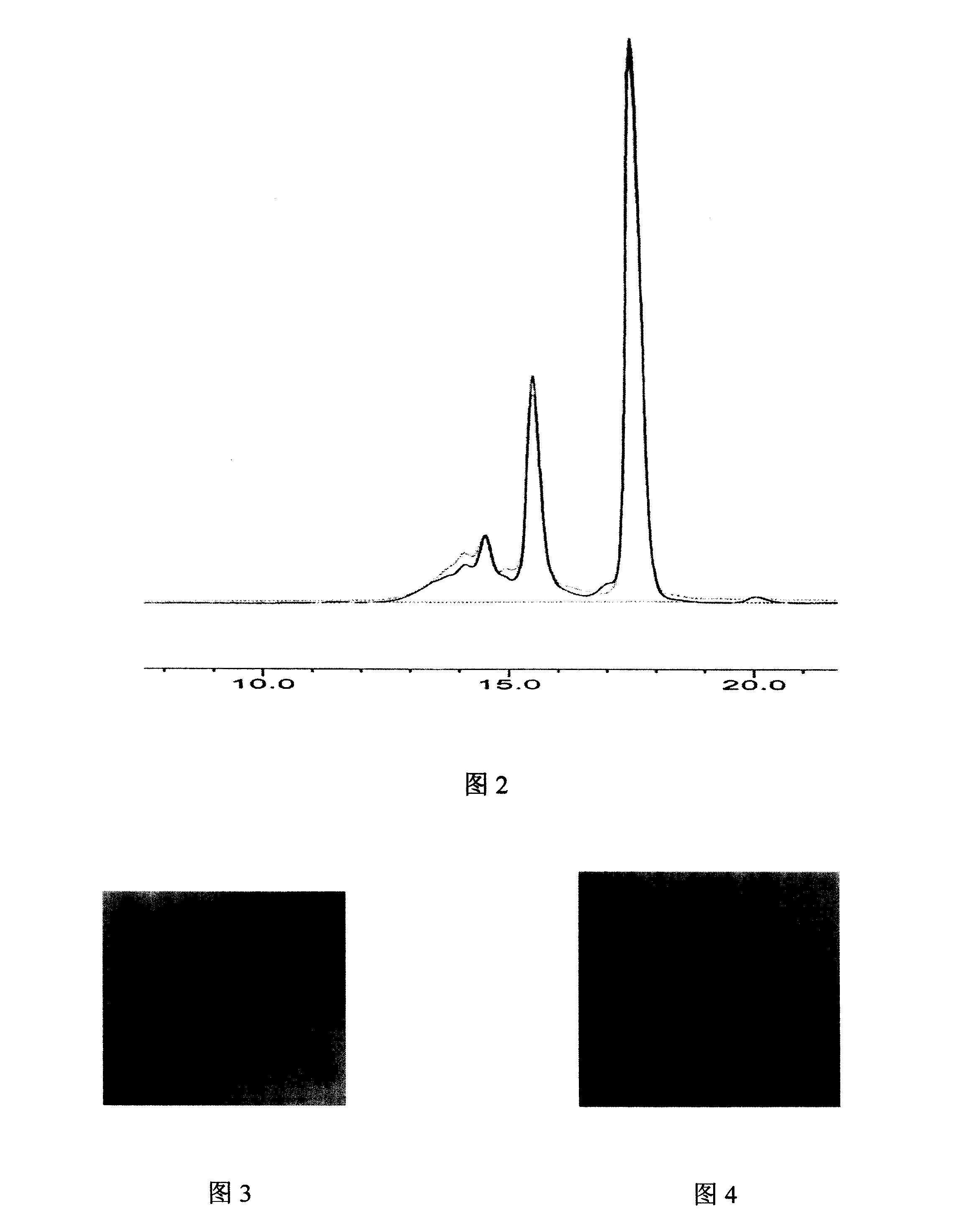
[0075] Embodiment: the preparation method of cerebroprotein hydrolyzate sodium chloride injection
[0076] Weigh 90 grams of sodium chloride, add 3000ml of water for injection to dissolve, add 1.5 grams of activated carbon, let it stand, decarbonize and filter, add water for injection to 8000ml, and make a sodium chloride solution; measure 1000ml of cerebroprotein hydrolyzate stock solution, of which, Aspartic acid 2.40~3.60mg / ml, threonine 0.21~0.39mg / ml, serine 0.21~0.39mg / ml, glutamic acid 3.20~4.80mg / ml, glycine 1.20~1.80mg / ml, alanine 2.40~3.60mg / ml, Valine 1.60~2.40mg / ml, Methionine 0.35~0.65mg / ml, Isoleucine 1.60~2.40mg / ml, Leucine 4.80~7.20mg / ml, Phenylalanine 1.60~2.40mg / ml, lysine 4.80~7.20mg / ml, histidine 1.04~1.56mg / ml, arginine 0.30~1.10mg / ml, tryptophan 0.35~0.65mg / ml and proline 1.60~2.40mg / ml.
[0077] Ultrafilter the above stock solution with an 8000 Dalton ultrafiltration membrane, add the filtrate to the sodium chloride solution; then add water for injecti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com