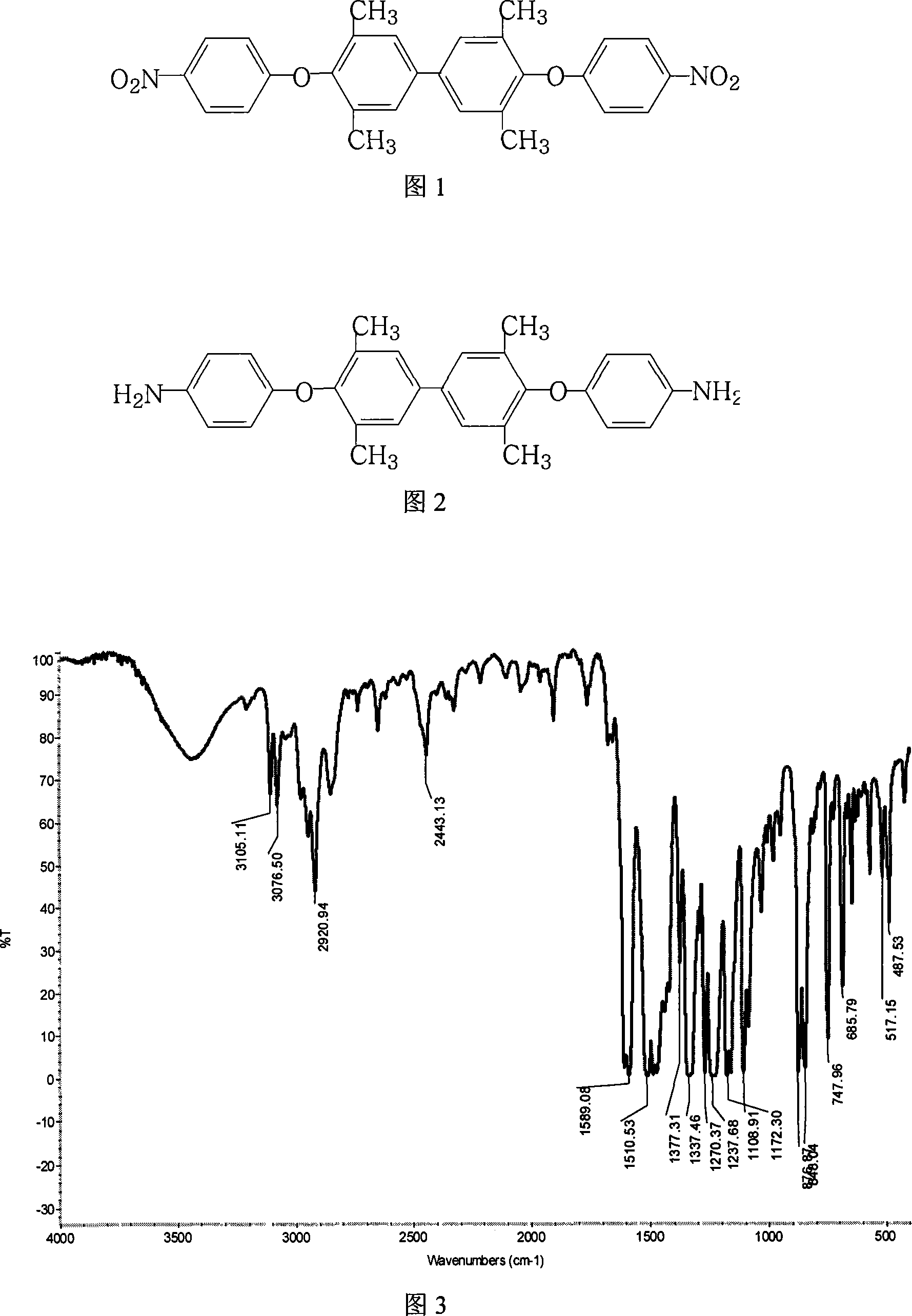
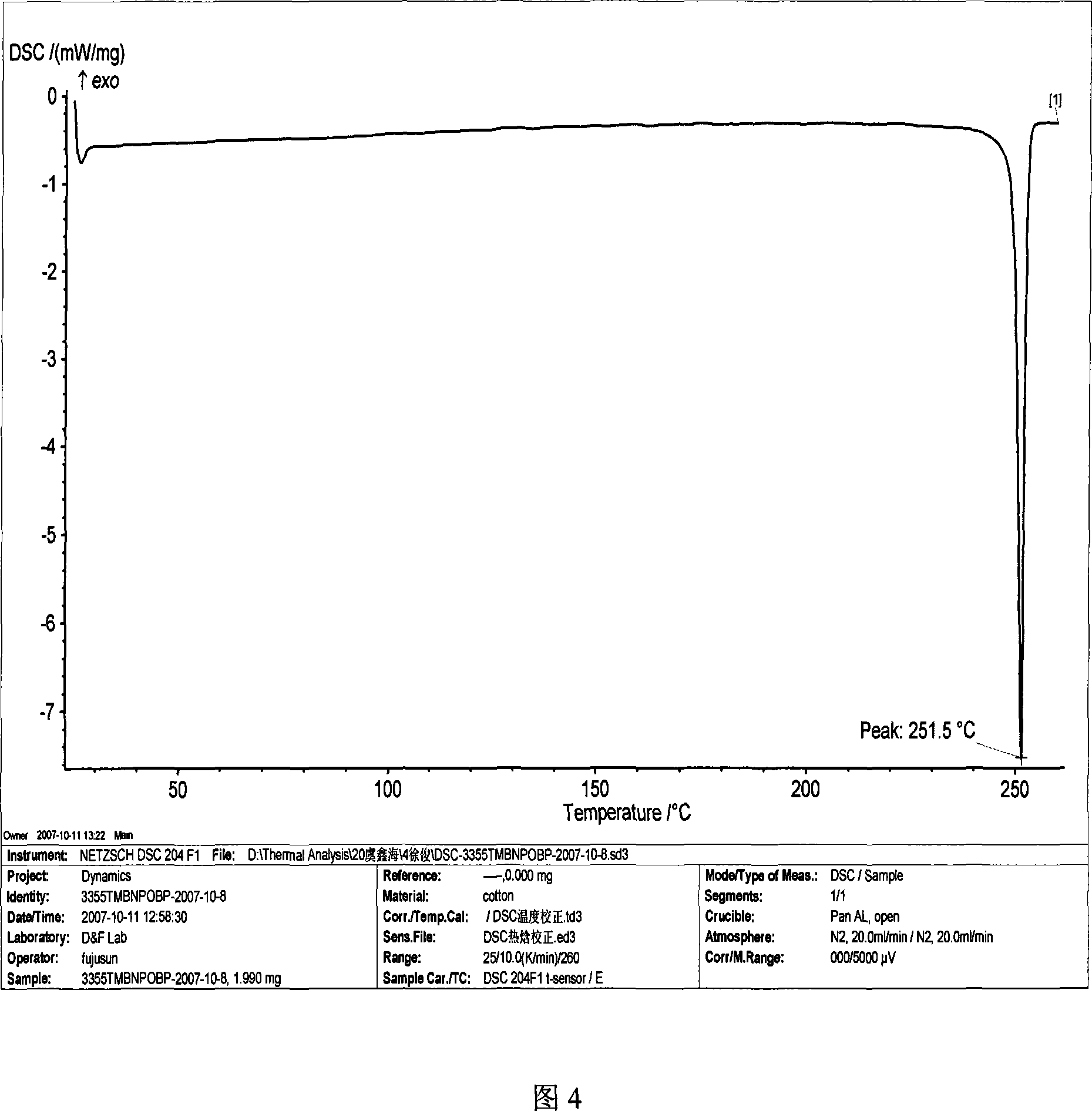
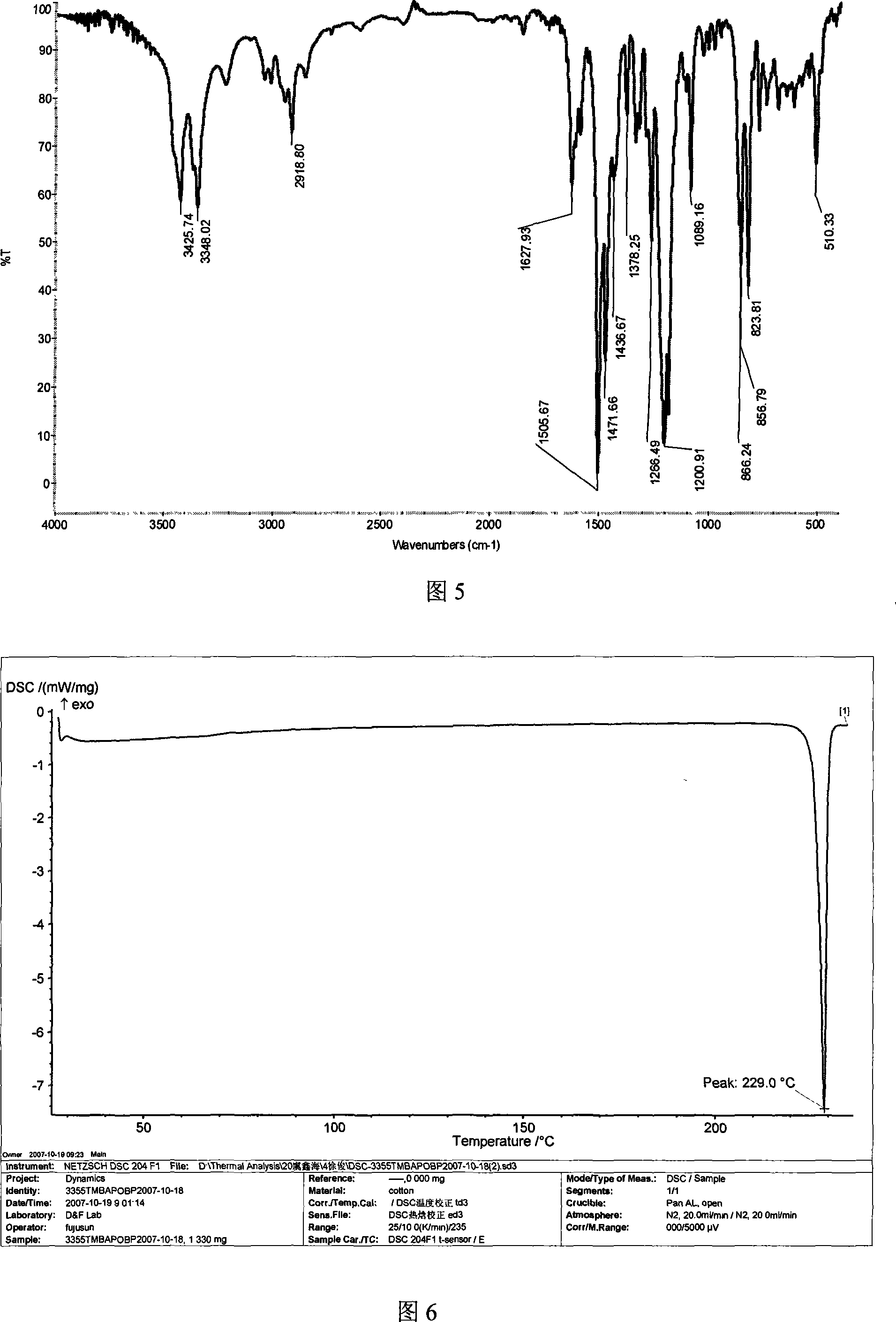
Method for preparing 4,4'-di(4-aminophenoxy)-3,3',5,5'-tetramethylbiphenyl
A technology of tetramethylbiphenyl and aminophenoxy, applied in 4 fields, can solve the problems of low total yield, unfavorable environmental protection, low purity and the like, and achieves high product yield and purity, low production cost and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] 24.2 grams (0.10 moles) of 3,3',5,5'-tetramethyl-4,4'-diphenol, 34.6 grams (0.22 moles) of 4-chloronitrobenzene, 110.4 grams (0.80 moles ) Potassium Carbonate, 700 milliliters of N, N-dimethylformamide and 180 milliliters of toluene were put into the reaction kettle, stirred, heated to reflux and separated water for 18 hours, concentrated the reaction solution, recovered the solvent for recycling, and cooled the reactant system , add water, separate out yellow-brown solid product, dry, obtain 45.1 grams of 4,4'-bis(4-nitrophenoxy)-3,3',5,5'-tetramethylbiphenyl, melting point is 251.5°C , with a purity of 99.5%. According to the actual amount of 1,4-bis(4-nitrophenoxy)benzene obtained and the theoretical amount (48.4 grams), 4,4'-bis(4-nitrophenoxy) was calculated )-3,3',5,5'-tetramethylbiphenyl yield was 93.2%. 4,4'-bis(4-nitrophenoxy)-3,3',5,5'-tetramethylbiphenyl Fourier Transform Infrared Spectrum (FTIR) diagram is shown in Figure 3, differential scanning calorimetr...
Embodiment 2
[0063] 24.2 grams (0.10 moles) of 3,3',5,5'-tetramethyl-4,4'-diphenol, 44.5 grams (0.22 moles) of 4-bromonitrobenzene, 55.2 grams (0.40 moles ) Potassium Carbonate, 150 milliliters of N, N-dimethylacetamide and 15 milliliters of dimethylbenzene are put into the reaction kettle, stir, and after heating and reflux water separation reaction for 2 hours, concentrate the reaction solution, reclaim the solvent to recycle, and cool the reactant system, adding water, a tan solid product was precipitated, purified and dried to obtain 31.7 grams of 4,4'-bis(4-nitrophenoxy)-3,3',5,5'-tetramethylbiphenyl, melting point It is 251.3 ℃, and the purity is 99.5%, according to the amount and theoretical amount (48.4 grams), the calculated yield of 4,4'-bis(4-nitrophenoxy)-3,3',5,5'-tetramethylbiphenyl was 65.5%.
Embodiment 3
[0065] 24.2 grams (0.10 moles) of 3,3',5,5'-tetramethyl-4,4'-diphenol, 28.4 grams (0.18 moles) of 4-chloronitrobenzene, 10.6 grams (0.10 moles ) Sodium carbonate, 40 milliliters of N-methyl-2-pyrrolidone and 15 milliliters of dichlorobenzene were put into the reaction kettle, stirred, heated to reflux and split water for 10 hours, concentrated the reaction solution, recovered the solvent for recycling, and cooled the reaction substance system, adding water, a tan solid product was separated out, purified and dried to obtain 40.1 grams of 4,4'-bis(4-nitrophenoxy)-3,3',5,5'-tetramethylbiphenyl, Melting point is 250.5 ℃, and purity is 99.0%, according to the amount and theoretical amount ( 43.5 g), the calculated yield of 4,4'-bis(4-nitrophenoxy)-3,3',5,5'-tetramethylbiphenyl was 92.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com