Vaccine containing composite adjuvant and preparation method thereof
An adjuvant and vaccine technology, applied in the biological field, can solve the problems of poor vaccine immune effect, weak immune response, and inability to effectively protect immunized animals, and achieve the effect of improving the intensity of immune response and improving response ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
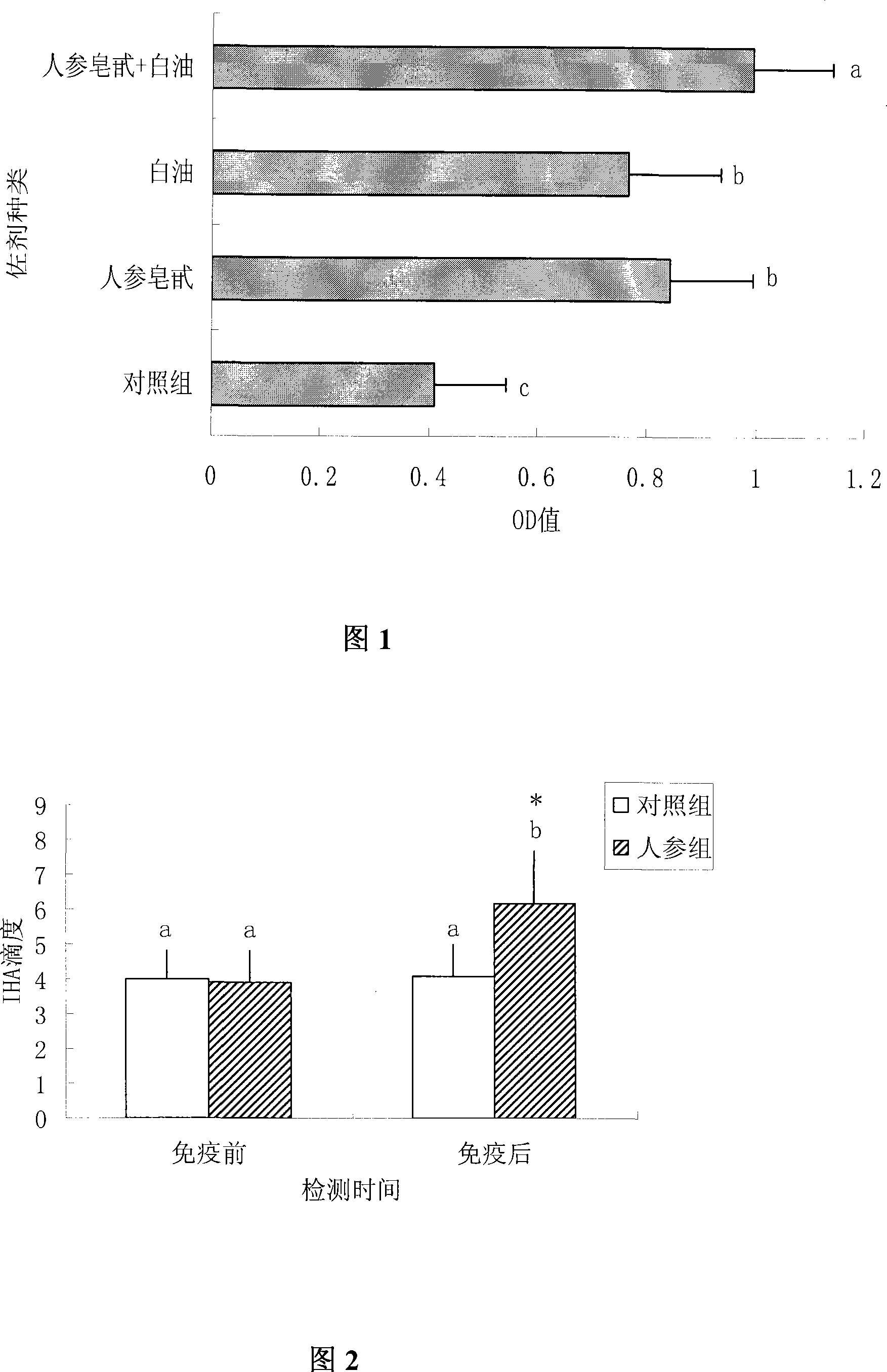
[0015] Example 1: The combination of ginsenoside and white oil enhances immunity of foot-and-mouth disease (FMD) vaccine.
[0016] 1. Materials and methods
[0017] 1. Experimental animals: 24 female ICR mice, purchased from Shanghai Experimental Animal Center.
[0018] 2. Antigen and adjuvant: the antigen is inactivated O-type foot-and-mouth disease virus (FMDV), provided by Lanzhou Veterinary Research Institute (LVRI), Chinese Academy of Agricultural Sciences. White oil was purchased from Hangzhou Chemical Materials Co., Ltd. Ginsenosides are products of Hongjiu Company.
[0019] 3. Vaccine preparation: first dissolve ginsenoside with a small amount of physiological saline, then mix with inactivated O-type foot-and-mouth disease virus (FMDV) antigen solution, and then fully mix with oil adjuvant to obtain the product.
[0020] 4. Immunization method: 24 mice were randomly divided into 4 groups, 6 mice in each group. Each mouse was injected intramuscularly with 0.2ml of t...
example 2
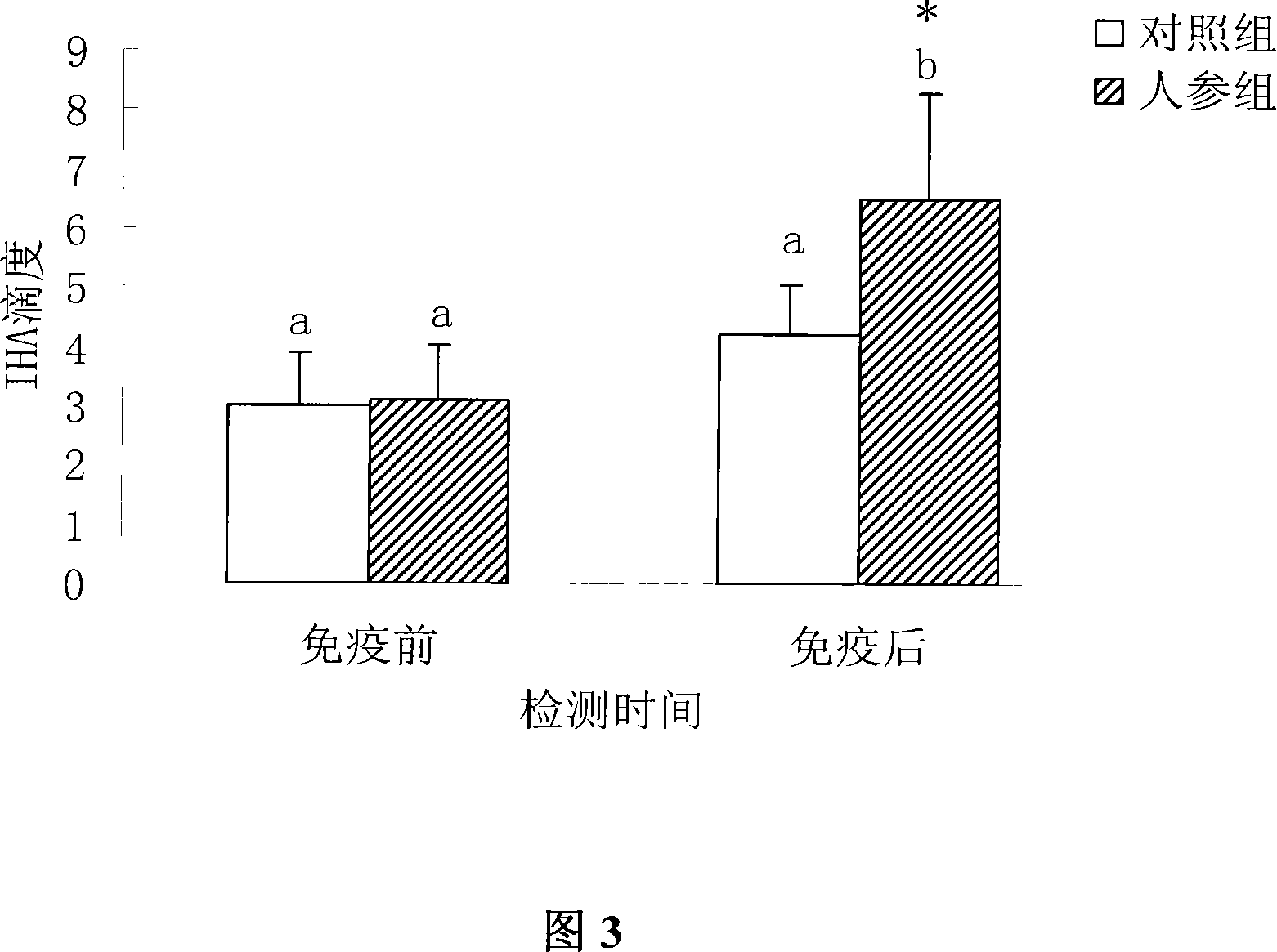
[0034] Example 2: Test of immunizing piglets with oil adjuvant foot-and-mouth disease vaccine (O type) containing ginsenoside (500 micrograms / ml)
[0035] 1. Materials and methods
[0036] 1. Experimental animals: 20 40-day-old piglets.
[0037] 2. Vaccine and adjuvant: Type O foot-and-mouth disease vaccine provided by Lanzhou Veterinary Research Institute (LVRI), Chinese Academy of Agricultural Sciences. Ginsenosides (GS) were provided by Hongjiu Company.
[0038] 3. Vaccine preparation: first dissolve ginsenosides with a small amount of normal saline, then add the ginsenoside solution to the vaccine and mix thoroughly so that the content of ginsenosides in each milliliter of vaccine is 0 (as a control) and 500 micrograms respectively.
[0039] 4. Immunization method: 20 piglets were randomly divided into 2 groups, 10 piglets in each group. Each pig was intramuscularly injected with 2ml of the vaccine once, blood was collected before and after the immunization, the serum w...
example 3
[0043] Example 3: Test of immunizing piglets with oil adjuvant foot-and-mouth disease vaccine (O type) containing ginsenoside (1000 micrograms / ml)
[0044] 1. Materials and methods
[0045] 1. Experimental animals: 22 40-day-old piglets.
[0046] 2. Vaccine and adjuvant: Type O foot-and-mouth disease vaccine provided by Lanzhou Veterinary Research Institute (LVRI), Chinese Academy of Agricultural Sciences. Ginsenosides (GS) were provided by Hongjiu Company.
[0047] 3. Vaccine preparation: first dissolve ginsenosides with a small amount of normal saline, then add the ginsenoside solution to the vaccine and mix thoroughly so that the content of ginsenosides in each milliliter of vaccine is 0 (as a control) and 1000 micrograms respectively.
[0048]4. Immunization method: 22 piglets were randomly divided into 2 groups, 11 piglets in each group. Each pig was intramuscularly injected with 2ml of the vaccine once, blood was collected before and after the immunization, the serum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com