Coagulation and fibrinolytic cascades modulator
A technology for promoting coagulation and prothrombin, which can be used in coagulation/fibrinolytic factors, blood diseases, and the determination/examination of microorganisms, which can solve problems such as less research.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
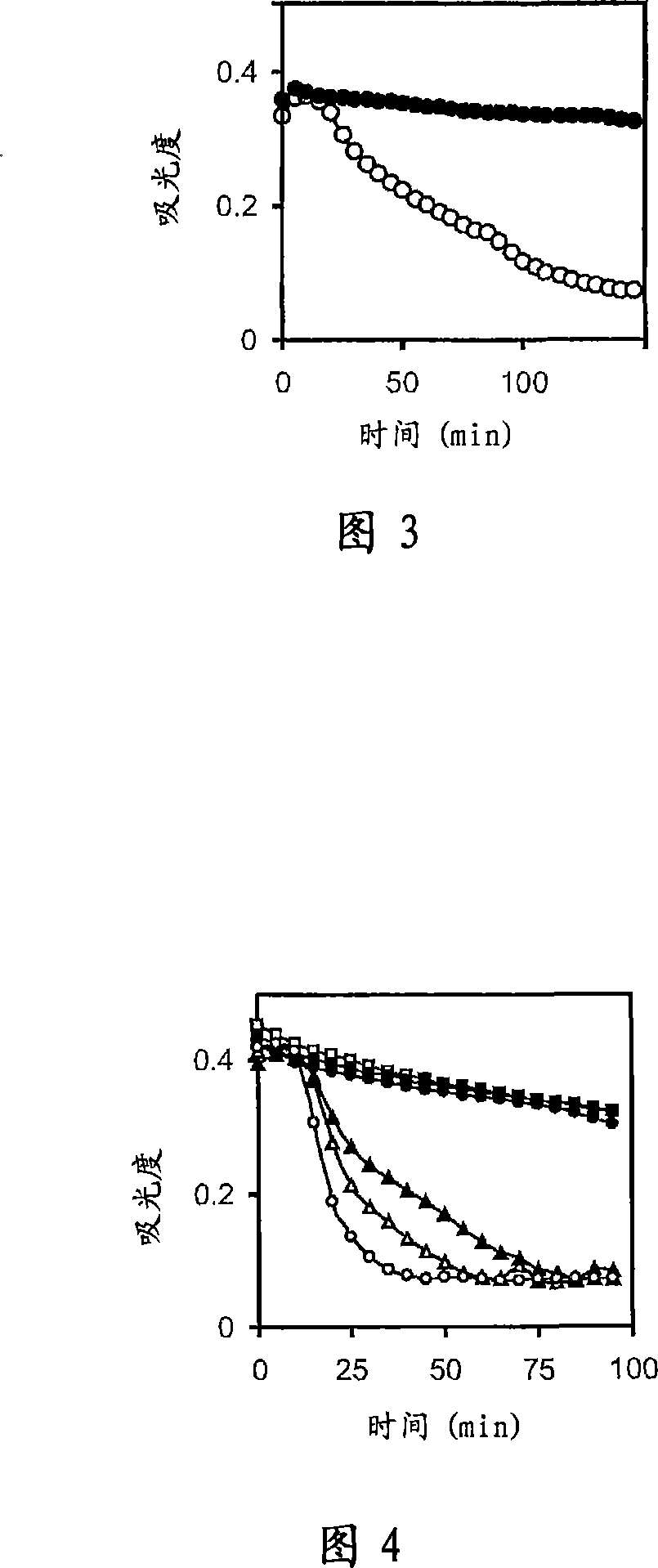
[0101] polyP affects the rate of plasma coagulation and the rate of fibrinolysis
[0102] The role of polyP in blood coagulation and fibrinolysis was initially investigated using a combined model of plasma coagulation and fibrinolysis. In this system, coagulation of citrated, collected normal human plasma was initiated by the addition of calcium chloride. Coagulation initiated in this way occurs via the intrinsic pathway of blood coagulation and is relatively slow without the addition of contact activators. Thrombin (the last enzyme in the coagulation cascade) proteolytically converts fibrinogen to fibrin, which spontaneously polymerizes to form a fibrin gel or clot. Coagulation of fibrin is associated with an increase in plasma turbidity, which can be easily monitored by measuring changes in absorbance at 405 nm. Addition of calcium ions concurrently with the addition of plasminogen activator to plasma results in activation of plasminogen to plasmin and subsequent lysis of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com