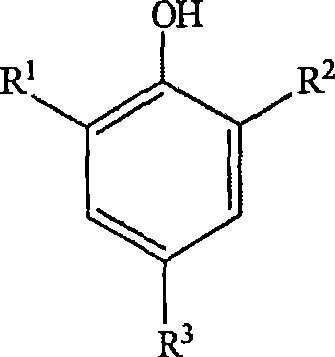
1-bromopropane having low acidity
A technology of bromopropane and alkaline conditions, applied in the field of 1-bromopropane products, can solve problems such as consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The preparation described in Comparative Example A was followed to form the final 1-bromopropane product. No 2,6-di-tert-butyl-4-methylphenol (BHT) was added during the preparation. 3 rounds of testing were performed to test the efficiency of the stabilizer in the 1-bromopropane product. In rounds 1 and 2, a certain amount of BHT was added to a known amount of 1-bromopropane product; in round 3, butylene oxide (abbreviated BOX in Table 1 below) was added to Added to a known amount of 1-bromopropane product. The stability tests described were performed on all three samples. No 1,2-epoxide appeared in run 1 or 2; no phenol was added in run 3, which is a comparative example. The amount of BHT or butylene oxide (BOX) added to the 1-bromopropane product in each run is listed in Table 1. The results for the 1-bromopropane product initially and after 10 and 30 days at 60°C are summarized in Table 1, expressed as the amount present in the final 1-bromopropane product.
[0...
Embodiment 2
[0071] The preparation described in Comparative Example A was carried out to form the final 1-bromopropane product; in addition, 2,6-di-tert-butyl-methyl Phenol (BHT, 50 kg) was added to the 1-bromopropane product mixture; it was seen by GC that a portion of the BHT (about 1000 ppm) was carried over in the purified 1-bromopropane product at the end of the distillation. At the end of step (5), enough butylene oxide is added to the purified 1-bromopropane product to achieve a butylene oxide concentration of about 450-500 ppm. The area percent detection of BHT in the final 1-bromopropane product by GC represented the presence of approximately 2 ppm BHT. The final 1-bromopropane product was subjected to the above acid test. The results for the 1-bromopropane product initially and after 10 days, 12 days, 25 days and 31 days at 60°C are summarized in Table 2, expressed as the amount present in the final 1-bromopropane product . The final 1-bromopropane product was also tested for...
Embodiment 3
[0077] The preparation described in Example 2 was followed including the addition of butylene oxide to form the final 1-bromopropane product. The amount of BHT added was 8 g of BHT per 1489 g of the 1-bromopropane product mixture; after the distillation step, no BHT was detected in the 1-bromopropane by GC nor was any new one detected in the 1-bromopropane product mixture compound of. The results for the 1-bromopropane product initially and after 10 and 30 days at 60°C are summarized in Table 3, expressed as the amount present in the final 1-bromopropane product. The final 1-bromopropane product was also tested for non-volatile residue as described above and the non-volatile residue was 3 ppm.
[0078] table 3
[0079] At the beginning of the stability test
[0080] * Acidity is reported in ppm HBr.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com