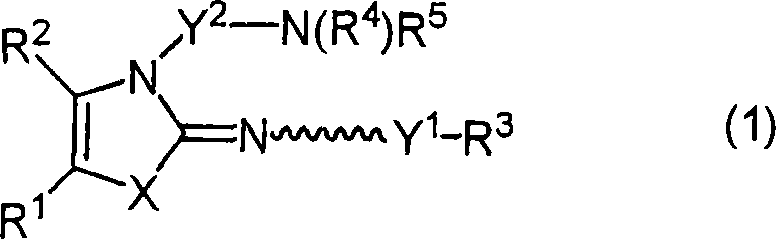Therapeutic agent for chronic obstructive pulmonary disease
A technology for chronic obstructive and pulmonary diseases, applied in allergic diseases, sensory diseases, respiratory diseases, etc., can solve the problems of different mechanisms of COPD activity and action, undisclosed prevention and/or treatment of COPD, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
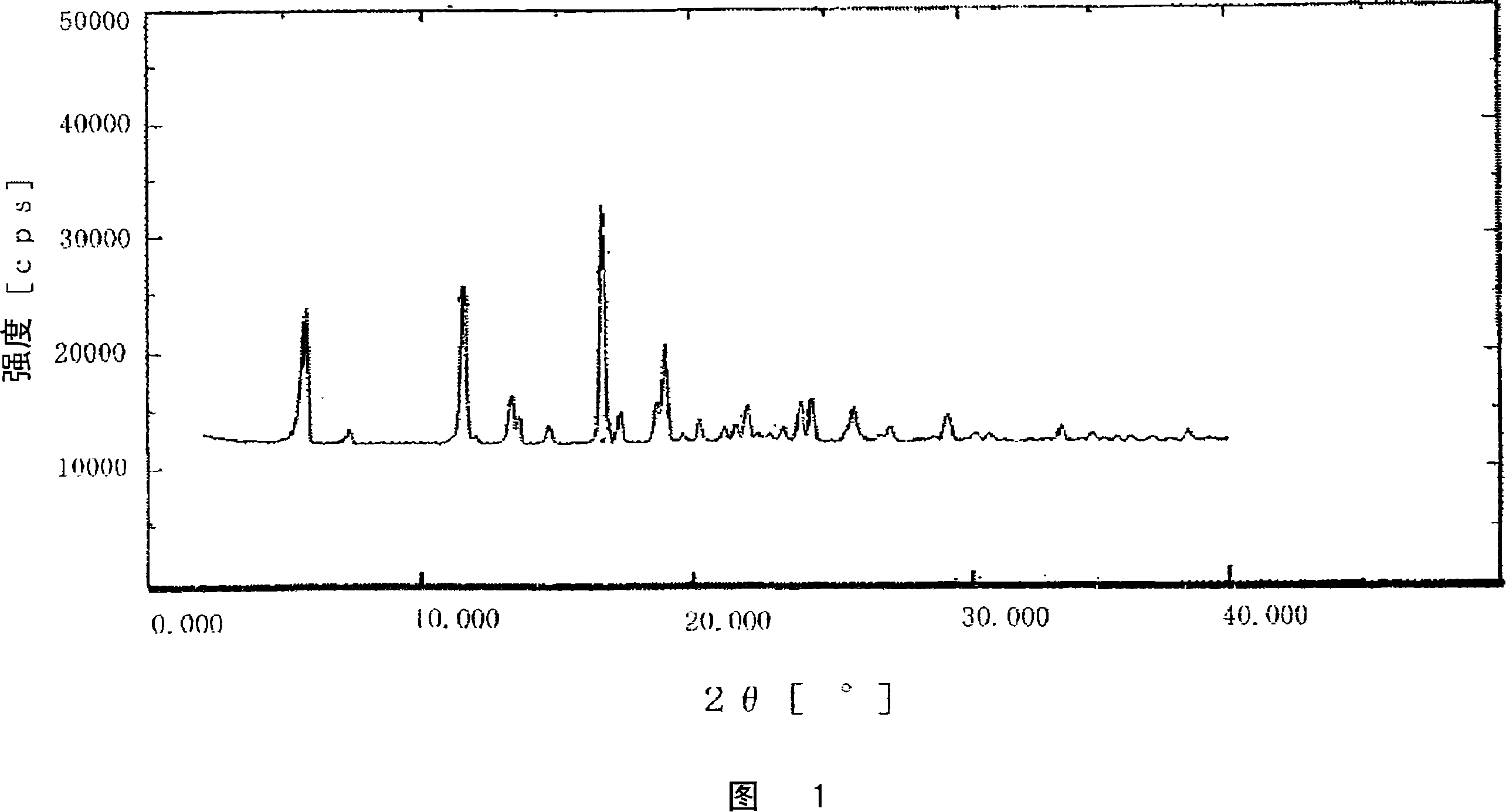
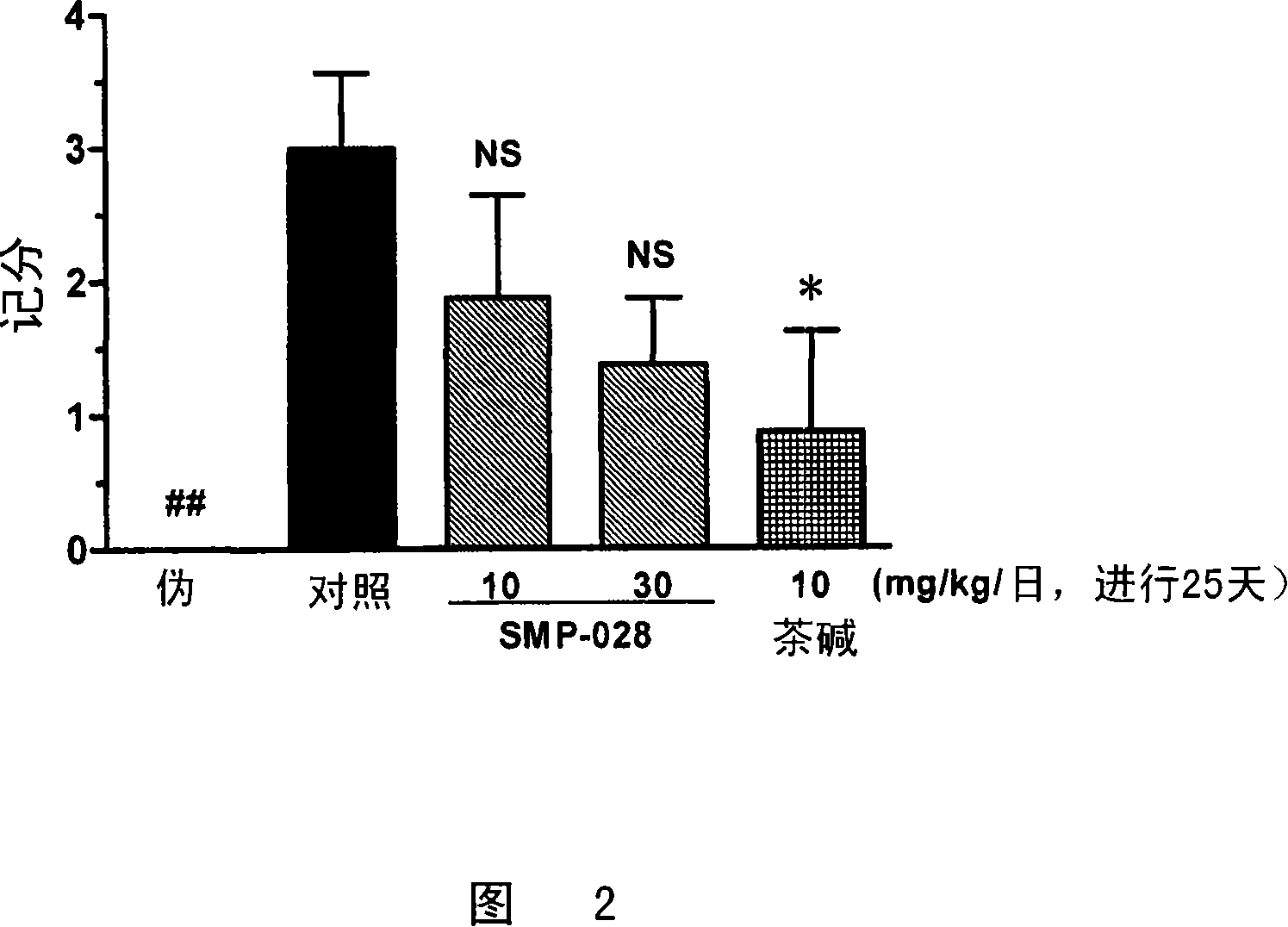
Image
Examples
Embodiment 1
[0145] N-{2-[2-[(3-fluorophenyl)imino]-4-(4-morpholinophenyl)-thiazol-3(2H)-yl]ethyl}-N'-methyl Urea (SMP-028)
[0146]
[0147] (1) To a solution of tert-butyl 2-(aminoethyl)carbamate (1.02g) in acetonitrile (20ml) was added dropwise 3-fluorophenylisothiocyanate (752mg), and the mixture was heated at 75°C 1 hour. The reaction mixture was concentrated in vacuo and crystallized from n-hexanol to give tert-butyl 2-{[(3-fluoroanilino)thiocarbonyl]amino}ethylcarbamate (1.81 g)
[0148] 1 H-NMR (CDCl 3 ): δ1.35(9H, s), 3.35(2H, m), 3.74(2H, m), 4.89(1H, bs), 6.99(3H, m), 7.37(1H, m), 7.81(1H, bs)
[0149](2) Under a nitrogen atmosphere, stir and heat the tert-butyl 2-{[(3-fluoroanilino)thiocarbonyl]amino}ethylcarbamate (1.81g) obtained above at 45°C, α-bromo - A mixture of 4'-morpholino-acetophenone (1.56 g) and ethanol (20 ml). After 1 hour, the resulting crystals were filtered to give {2-[2-[(3-fluorophenyl)imino]-4-(4-morpholinophenyl)-thiazol-3(2H)-yl]ethyl } tert-But...
Embodiment 2
[0156] N-{2-[2-[(3-fluorophenyl)imino]-4-(4-morpholinophenyl)-thiazol-3(2H)-yl]ethyl}-N'-methyl Urea salt
[0157] (1) N-{2-[2-[(3-fluorophenyl)imino]-4-(4-morpholino-4-ylphenyl)-1,3-thiazole-3(2H)- Synthesis of ]ethyl}-N'-methylurea hydrobromide
[0158] To N-{2-[2-[(3-fluorophenyl)imino]-4-(4-morpholino-4-ylphenyl)-1,3-thiazol-3(2H)-yl] To a suspension of ethyl}-N'-methylurea (455 mg) in chloroform (50 ml) was added 25% HBr / AcOH (0.32 g), and the dissolved mixture was stirred for 30 minutes. Diethyl ether was added thereto after removal of the solvent, and the precipitate was filtered to obtain crystals (520 mg) with a melting point of 191-193°C.
[0159] (2) N-{2-[2-[(3-fluorophenyl)imino]-4-(4-morpholino-4-ylphenyl)-1,3-thiazole-3(2H)- Synthesis of ]ethyl}-N'-methylurea benzenesulfonate
[0160] Following the same procedure as the above method, benzenesulfonic acid (158 mg) was used to obtain crystals (610 mg) having a melting point of 153.5-156°C.
[0161] (3) N-{2-...
Embodiment 3
[0168] N-{2-[2-[(3-fluoro-4-hydroxyphenyl)imino]-4-(4-morpholinophenyl)-thiazol-3(2H)-yl]ethyl}-N '-methylurea
[0169]
[0170] (1) Phenyl chlorothionoformate was added to a toluene solution of 4-amino-2-fluorophenol, and 30 minutes later, 1N aqueous sodium hydroxide solution was added thereto. The mixture was stirred at room temperature for 3 hours, after which tert-butyl 2-(aminoethyl)carbamate was added and the mixture was stirred overnight to give 2-{[(3-fluoro-4-hydroxyanilino)thiocarbonyl ]Amino}ethylcarbamate tert-butyl ester.
[0171] (2) According to the steps similar to those in Example 1, use tert-butyl 2-{[(3-fluoro-4-hydroxyanilino)thiocarbonyl]amino}ethylcarbamate obtained in (1) above and α-bromo-4'-morpholino-acetophenone to give the title compound.
[0172] 1 H-NMR (DMSO-d6): δ2.46 (3H, d, J=4.7), 3.17-3.24 (6H, m), 3.72-3.76 (6H, m), 6.01 (1H, s), 6.63-6.66 (1H, m), 6.75-6.78 (1H, m), 6.87-6.93 (1H, m), 6.99 (2H, d, J=8.85) and 7.29 (2H, d, J=8.85). ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com