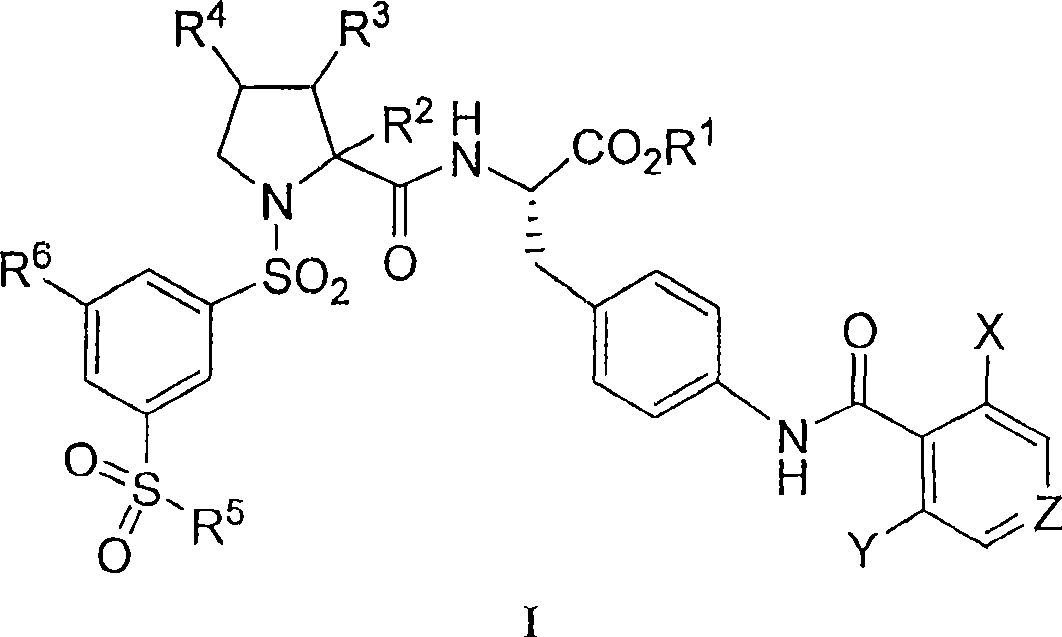
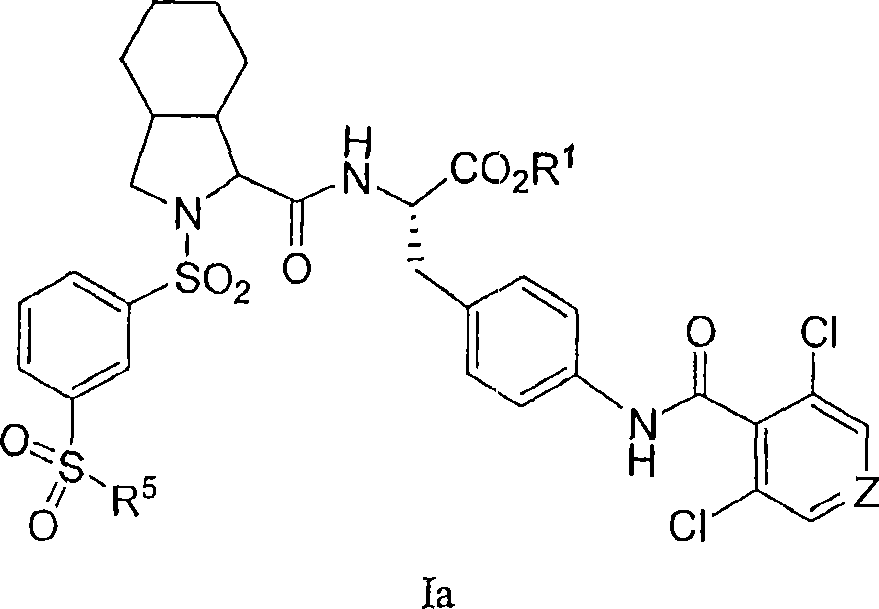
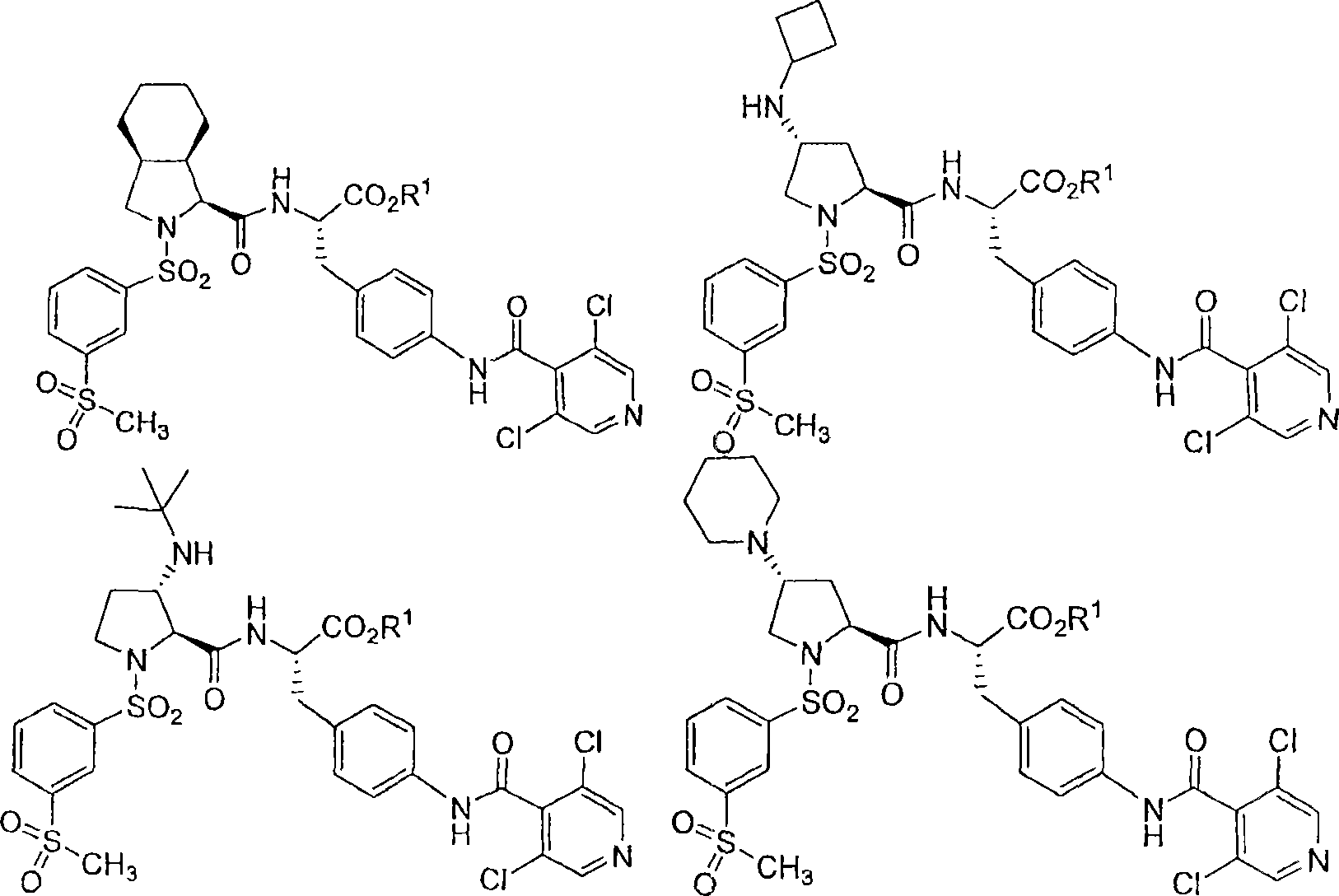
Vla-4 antagonists
An alkyl, selected technology, applied in the field of VLA-4 antagonists, can solve the problems of short half-life, unsuitable for oral administration, poor pharmacokinetic properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] (R, S, S)-N-{N-[(3-methylsulfonylphenyl)sulfonyl]-4(R)-cyclobutylamino-(L)-proline Aminoacyl}-4-[(3′,5′-dichloro-isonicotinyl)amino]-(L)-phenylalanine ethyl ester
[0137]
[0138] Step A: N-BOC-4(R)-cyclobutylamino-L-proline, methyl ester
[0139]
[0140] To a solution of N-BOC-cis-hydroxy-L-proline methyl ester (60g, 0.25mol) and N,N-diisopropylethylamine (100mL, 0.57mol) in dichloromethane (800mL) Trifluoromethanesulfonic anhydride was added within 45 minutes at -20°C. After continued stirring at -20°C for 45 minutes, cyclobutylamine (55 g, 0.78 mol) was added in one portion and the reaction was allowed to warm to room temperature overnight. The reaction was quenched with 1N NaOH (250 mL) and saturated aqueous NaHCO3 (250 mL). The organic layer was separated, dried with brine, dried over magnesium sulfate, filtered and concentrated, and the residue was purified by flash column chromatography on silica gel with CH 2 Cl 2 to EtOAc to 5% MeOH / EtOAc) to ...
Embodiment 2
[0149]
[0150] (R, S, S)-N-{N-[3-methylsulfonylphenyl)sulfonyl]-4(R)cyclobutylamino-(L)-prolyl Base}-4-[(3′,5′-dichloro-isonicotinyl)amino]-(L)-phenylalanine
[0151] To N-{N-[(3-methylsulfonylbenzenesulfonyl)-cyclobutylamino-(L)-prolyl}-4-[(3′,5′-dichloro-isonicotinyl) To a solution of amino]-(L)-phenylalanine ethyl ester (compound of Example 1, 4.794 g, 6.378 mmol) in 40 mL of acetonitrile was added 15.93 mL of 1N NaOH. After stirring at room temperature for 2 h, the reaction was quenched with 8 mL of 2N HCl to make the reaction solution slightly acidic. The reaction was diluted with 450 mL of THF / EtOAc (1:3 v / v) and washed with water (100 mL×2). After evaporation of the solvent, the residue was dissolved in 100 mL of acetonitrile and lyophilized to afford 4.6 g (99%) of the title product. 1 H NMR (500MHz, CD 3 OD): δ8.62(s, 2H), 8.3 3(s, 1H), 8.20(d, 1H), 7.90(d, 1H), 7.76(t, 1H), 7.60(d, 2H), 7.34( d, 2H), 4.49(dd, 1H), 4.44(dd, 1H), 3.70(dd, 1H), 3.63(m, 1H), ...
Embodiment 3
[0153]
[0154] N-{N-[3-methylsulfonylphenyl)sulfonyl]-3(S)-tert-butylamino-(L)-prolyl}-4- [(3′,5′-Dichloro-isonicotinyl)amino]-(L)-phenylalanine, ethyl ester
[0155] Step A: N-[(-methylsulfonylphenyl)sulfonyl]-3(S)-hydroxy-(L)-proline, methyl ester
[0156] To a solution of (3S)-hydroxy-(L)-proline (Acros, 1.223 g, 9.324 mmol) and sodium carbonate (2.08 g, 19.63 mmol) in 30 mL of water was added powdered 3-methyl Sulfonylbenzenesulfonyl chloride (2.5 g, 9.815 mmol). After stirring at room temperature overnight, the reaction mixture was acidified with concentrated hydrochloric acid (pH=3), and the product was extracted with ethyl acetate (3×30 mL). Dry organic extracts (MgSO 4 ), filtered and concentrated to dryness. The residue was then dissolved in dichloromethane (10 mL) and methanol (10 mL), and trimethylsilyldiazomethane (2M in ether) was added at 0 °C until the color persisted to yellow. After stirring at room temperature for 15 minutes, the mixture was con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com