4'-substituted benzyloxy-phenyl butadiene derivatives and preparation and uses thereof
A technology of dimethoxyphenyl and benzyloxy is applied in the field of preparing antitumor drugs, which can solve the problems of unsatisfactory, limited universal applicability of drugs, and malignant killing of normal cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
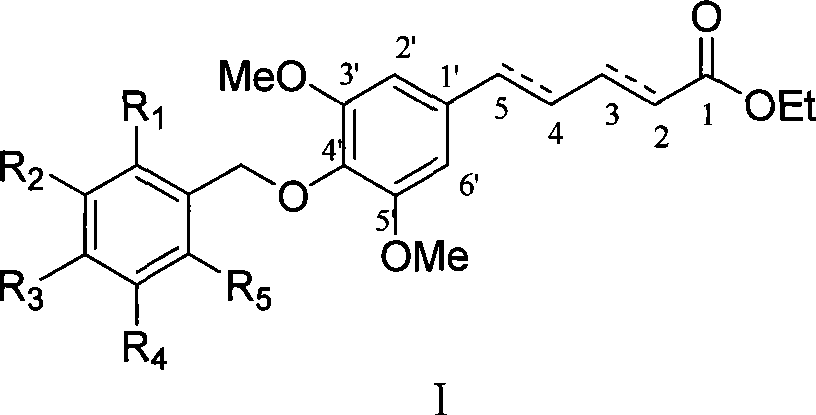
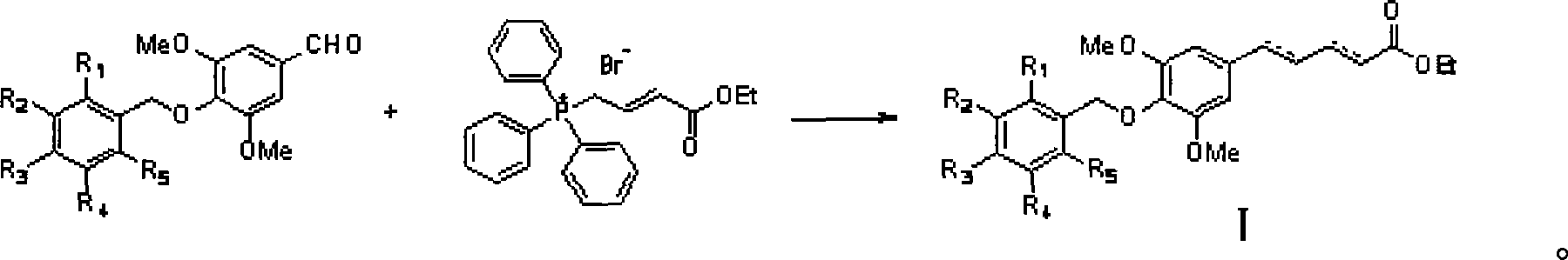
[0034] Example 1 : Preparation of isomer 5-(4'-p-ethoxybenzyloxy-3',5'-dimethoxyphenyl)penta-2,4-dienoic acid ethyl ester (I-a~I-c)
[0035]
[0036]This example relates to a class of 5-(4'-substituted benzyloxy-3',5'-dimethoxyphenyl)penta-2,4-bis having cytotoxic activity as shown in formula (I). General synthesis of ethyl enoate derivatives. Specifically, it relates to the synthesis of ethyl 5-(4'-p-ethoxybenzyloxy-3',5'-dimethoxyphenyl)penta-2,4-dienoate. Sodium (85 mg, 3.67 mmol) and absolute ethanol (2 ml) were stirred and reacted under argon protection. When the mixed solution was uniform, it was lowered to room temperature, 10 ml of tetrahydrofuran was added, and ethyl bromocrotonate was added. Ester triphenylphosphine salt (1.84 g, 4.04 mmol) was a yellow turbid liquid, and then added 3,5-dimethoxy-4-p-ethoxybenzylbenzaldehyde (1.16 g, 3.67 mmol) under stirring ), stirred at room temperature for 2 hours, added a small amount of water to quench the reaction, adjus...
Embodiment 2
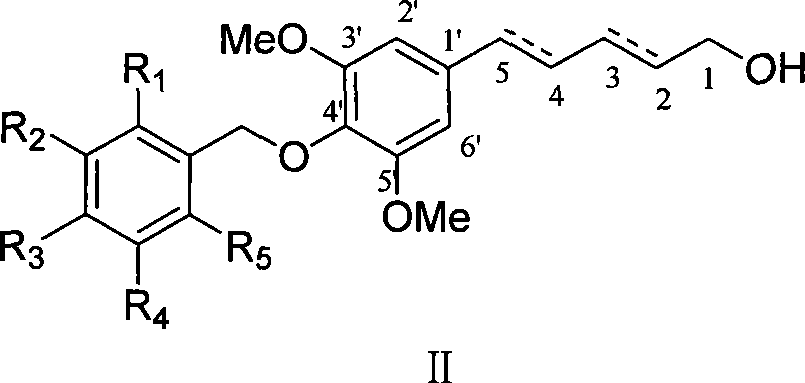
[0041] Example 2 : Compound II-a is (2E,4E)-5-(4'-p-ethoxybenzyloxy-3',5'-dimethoxyphenyl)penta-2,4-diene-1- Preparation of Alcohol
[0042]
[0043] This example relates to a class of 5-(4'-substituted benzyloxy-3',5'-dimethoxyphenyl)penta-2,4-bis having cytotoxic activity as shown in formula (II) General synthesis of en-1-ol derivatives. Specifically it relates to the synthesis of 5-(4'-p-ethoxybenzyloxy-3',5'-dimethoxyphenyl)penta-2,4-dien-1-ol. Under ice-cooling, lithium aluminum hydride (160 mg, 4.2 mmol) and aluminum trichloride (196 mg, 1.4 mmol) were added to anhydrous tetrahydrofuran, stirred for 5 minutes, and then raw material I-a (486 mg, 1.2 mmol mol) of anhydrous tetrahydrofuran solution was added dropwise to the reaction system, the temperature was controlled below 10°C, stirred for 2 hours, and a small amount of water was added dropwise to quench the reaction. The recovered tetrahydrofuran was added to dilute the reaction system and filtered, and the fi...
Embodiment 3
[0045] Example 3 : Preparation of Compounds II-b, II-c and II-d
[0046] According to the method of Example 2, using the compound of formula (I) of the corresponding configuration as a raw material, the conjugated diene compounds II-b and II-c were obtained through reduction, and the product II in which one of the double bonds was reduced was obtained. -d.
[0047] Compound II-b: yield: 51.3%; white solid; mp 93-95°C; Rf (n-hexane / ethyl acetate 5:2) 0.18; 1 H NMR (400MHz, CDCl 3 ): δ7.00 (2H, d, J=8.4Hz, H-3″, 7″), 6.90 (1H, d, J=15.2Hz, H-3), 6.74 (1H, d, J=8.8Hz , H-4″, 6″), 6.56 (2H, s, H-2′, 6′), 6.39 (1H, d, J=11.2Hz, H-5), 6.18 (1H, d, J=11.2 Hz, H-4), 5.91 (1H, dt, J=15.2Hz, H-2), 5.65 (2H, s, H-1′), 4.12 (2H, d, J=7.6Hz, H-1) , 3.95 (2H, q, J=7.6Hz, OCH 2 CH 3 -5″), 3.87 (6H, s, OCH 3 -3', 5'), 1.36 (3H, t, J=7.2Hz, OCH 2 CH 3 -5″); ESI-MS m / z[M+H] + 371.
[0048] Compound II-c: yield: 43.4%; white solid; mp 66-68°C; Rf (n-hexane / ethyl acetate 5:2) 0.20; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com