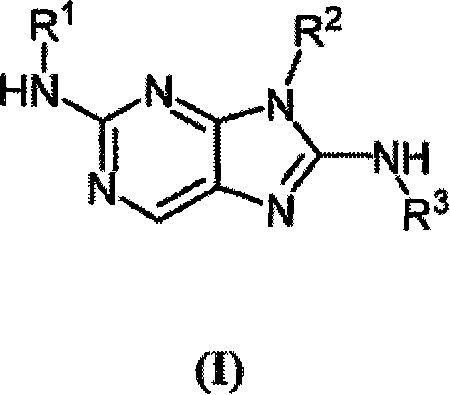
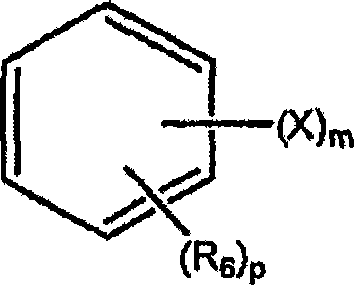Haloaryl substituted aminopurines, compositions thereof, and methods of treatment therewith
A kind of aryl and compound technology, applied in halogenated aryl substituted aminopurine, its composition and its treatment field, can solve problems such as incomplete response in treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 51
[0357] Example 5.1: Synthesis of 4-({8-[(2,6-difluorophenyl)amino]-9-cyclopentylpurin-2-yl)amino)trans-cyclohexyl-1-ol
[0358]
[0359] 1.(2-Chloro-5-nitropyrimidin-4-yl)cyclopentylamine
[0360] 2,4-Dichloro-5-nitropyrimidine (10.31mmol, 2g) and cyclopentylamine (10.31mmol, 1.02mL) were dissolved in THF (60mL) and cooled to -78°C. N,N-diisopropylethylamine (10.31 mmol, 1.8 mL) was added dropwise. The reaction mixture was stirred at -78°C for about 45 minutes. The cooling bath was removed, and the reaction mixture was stirred at room temperature for about 16 hours. After removing the solvent, the residue was dissolved in EtOAc again and washed with water and brine. MgSO for organic layer 4 Dry and evaporate the solvent. Column chromatography (SiO 2 , 9:1 n-hexane / ethyl acetate) for purification to obtain the desired product (2.11 g, yield 84%). ES-MS: 242 (M+1). When replacing the above cyclopentylamine with amine hydrochloride, use 2-3 equivalents of N,N-diisopropylethylamine ...
Embodiment 52
[0367] Example 5.2: Synthesis of trans-(4-aminocyclohexyl){8-[2,4-difluorophenyl]amino]-9-cyclopentylpurin-2-yl}amine
[0368]
[0369] 1. Trans-(4-aminocyclohexyl){8-[2,4-difluorophenyl]amino]-9-cyclopentylpurin-2-yl}amine
[0370] N-[4-({8-[(2,4-difluorophenyl)amino]-9-cyclopentylpurin-2-yl}amino)trans-cyclohexyl](tert-butoxy)methyl The amide (0.71 mmol, 375 mg) was dissolved in ethanol (6 mL) and cooled to 0°C. Acetyl chloride (3 mL) was added dropwise, the reaction was carried out at room temperature, and the mixture was stirred overnight. The precipitate was filtered off, washed with ether, and dried under high vacuum to obtain 372 g (98% yield) of trihydrochloride. ES-MS: 428 (M+1).
[0371] Alternatively, N-[4-({8-[(2,4-difluorophenyl)amino]-9-cyclopentylpurin-2-yl}amino)trans-cyclohexyl](tert-butyl The oxy) formamide was dissolved in 9 mL of dichloromethane, followed by 2.25 mL of TFA. The reaction mixture was stirred for about 2 hours. The solvent was removed in vacuo a...
Embodiment 53
[0372] Example 5.3: Synthesis of 8-(2-fluorophenylamine)-2-(4-methoxyphenylamine)-9-(trans-4-(methylamino)cyclohexyl)-9H-purine
[0373]
[0374] The Boc-protected amine (481 mg, 0.88 mmol) was dissolved in THF (6 mL), and lithium aluminum hydride (1.0 M in THF, 2.64 mL, 2.64 mmol) was added. The reaction mixture was heated to 65°C overnight. The reaction mixture was cooled to 0°C, and water was added dropwise to stop the reaction until no more hydrogen gas appeared. The precipitate was filtered off and washed thoroughly with ethyl acetate. The solvent was removed in vacuo, and the residue was purified by semi-preparative HPLC (20% acetonitrile / water (0.1% TFA)→80% acetonitrile / water (0.1% TFA), 30 min) to obtain 191 mg of product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com