Q type non-viral vector and pharmaceutical composition containing the same
A composition and drug technology, applied in the direction of drug combination, the use of carriers to introduce foreign genetic material, and non-effective ingredients of polymer compounds, etc., can solve the problems of toxic side effects of killing effect, immunogenicity problems, neutralization and failure of toxin killing activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
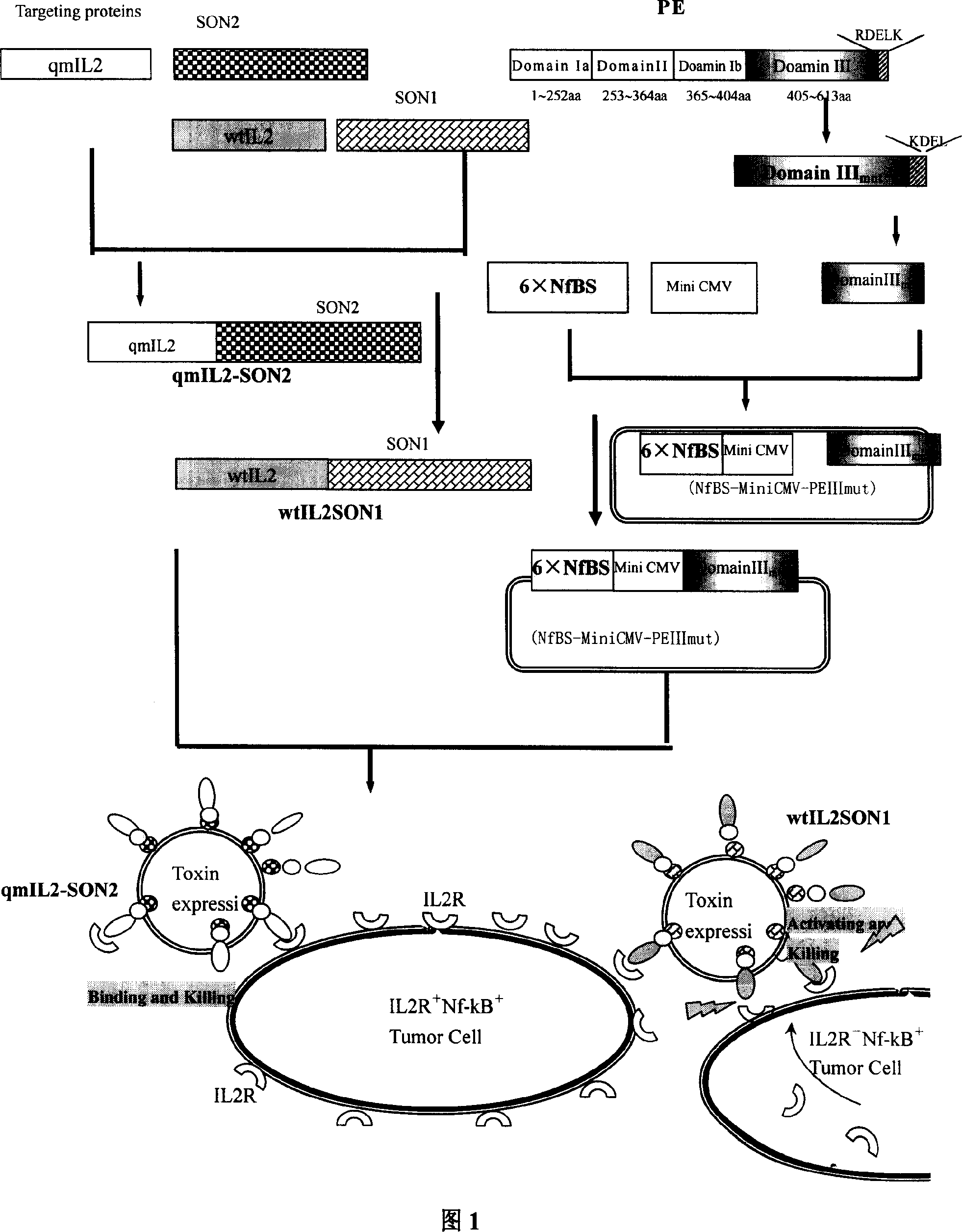
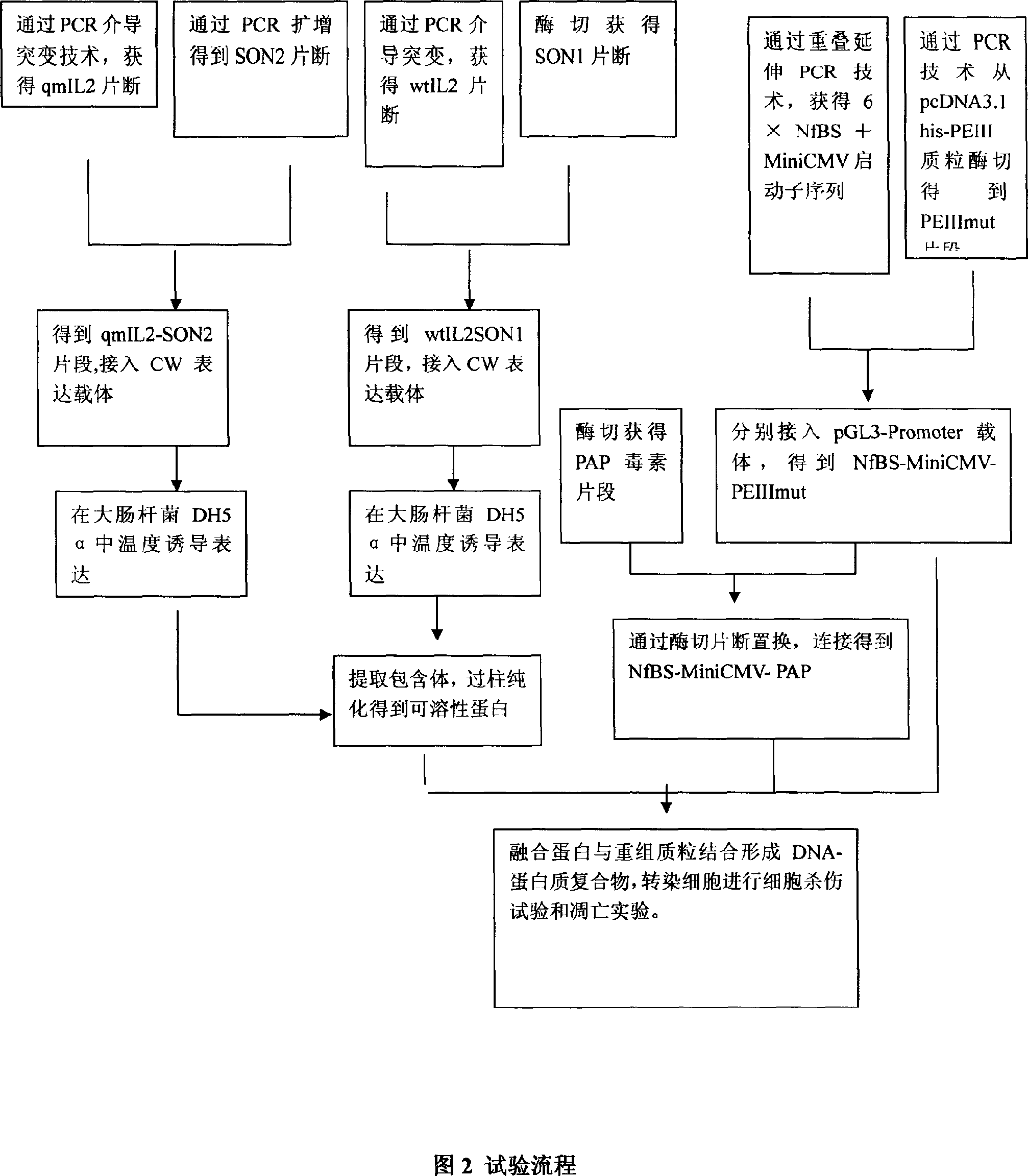
[0055] Example 1 Construction of fusion gene recombinant qmIL2-SON2 and wtIL2SON1
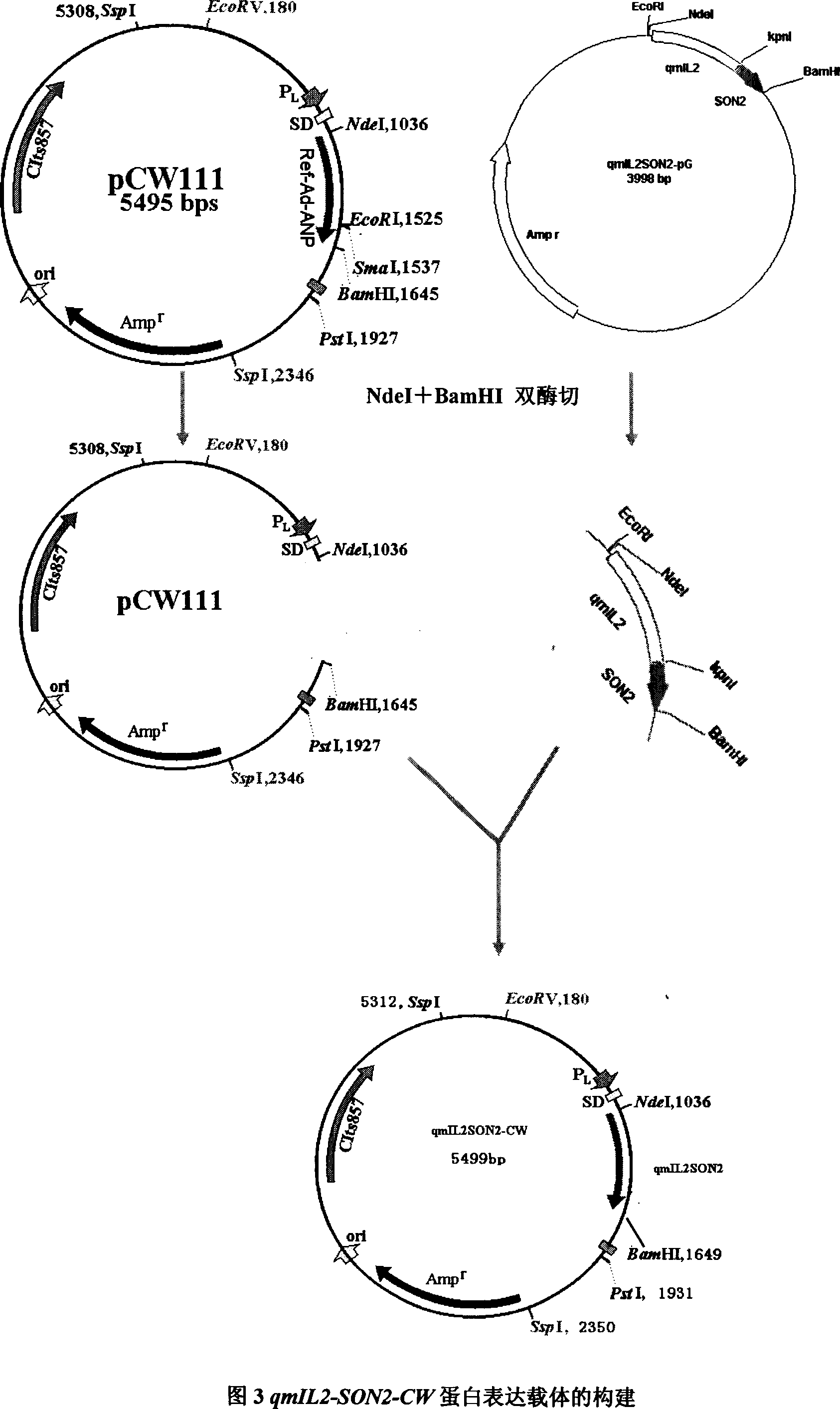
[0056] 1.1 Construction of qmIL2-SON2 sequence:
[0057] The IL2 fragment was amplified using the CW120 containing IL2 sequence of Dr. Chen Weijing in our laboratory, and after sequencing, it was found that compared with the wild type, there are two mutations in the IL2 template sequence that affect amino acid coding. It is necessary to design mutation primers for these mutation sites , Perform back mutation. Finally, the 58 and 125 Cys coding codons of the IL2 sequence were mutated into Ser coding codons, and the remaining sequences were the same as the wild-type sequence.
[0058] While mutating the IL2 sequence, use the upstream and downstream full-length primers FF and RF to introduce the restriction sites EcoRI and Nde I required for cloning at the 5'end of the IL2 sequence, respectively; at the 3'end of the IL2 sequence Introduce the thrombin cleavage site as the Linker sequence inside the fu...
Embodiment 2
[0065] Example 2 Induced expression of fusion gene qmIL2-SON2 or wtIL2SON1, extraction and detection of inclusion bodies
[0066] 2.1 Temperature induction and extraction of inclusion bodies
[0067] 2.1.1 Large volume culture
[0068] The constructed expression recombinant was transformed into Escherichia coli DH5α, and the transformed product was spread on an LB plate containing ampicillin and cultured overnight at 32°C. Take out several 2-3mm single colonies from the plate of the recipient strain, transfer them to a test tube containing 5ml of LB (containing appropriate antibiotics) culture solution, and culture them with vigorous shaking at 37°C overnight until they become turbid. On the second day, the overnight bacteria were also transferred to a medium bottle (150-200ml containing appropriate antibiotics) LB culture medium at a ratio of 1-2%, and cultured with vigorous shaking at 30-32°C overnight until turbid. On the third day, transfer the overnight bacteria to 6 large bo...
Embodiment 3
[0086] Example 3 Purification of protein qmIL2-SON2 or wtIL2SON1 from E. coli inclusion bodies
[0087] 1. Inclusion bodies are in the proportion of 20ml per gram. The inclusion bodies were dissolved with inclusion body dissolving solution (50mM Tris-HCl, 2mM EDTA, 100mM NaCL, 10mM DTT, 7M guanidine hydrochloride, pH9.0), and passed through a 0.45μm pore filter membrane.
[0088] 2. Reverse phase column: use AKTA medium pressure liquid chromatography system, chromatography column Waters AP1 column packing SOURCE 15RPC, flow rate 3ml / min. Buffer: starting buffer A solution: 10% acetonitrile, 0.05% TFA, starting buffer B solution: 90% acetonitrile, 0.05% TFA.
[0089] Gradient: 100% solution A maintains 3 column volumes to elute unbound proteins and impurities; the concentration of solution B rises to 100% within 15 minutes and maintains 2 column volumes. Collect the eluted protein in 8ml.
[0090] After qmIL2-SON2 was purified by reversed-phase column, the purity of the protein was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com