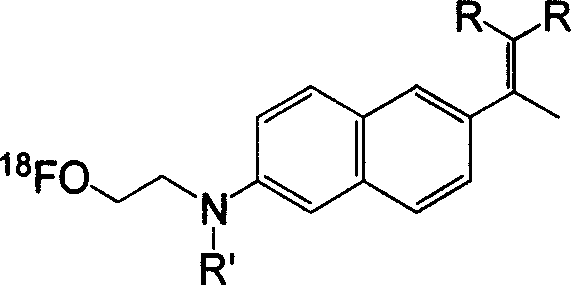
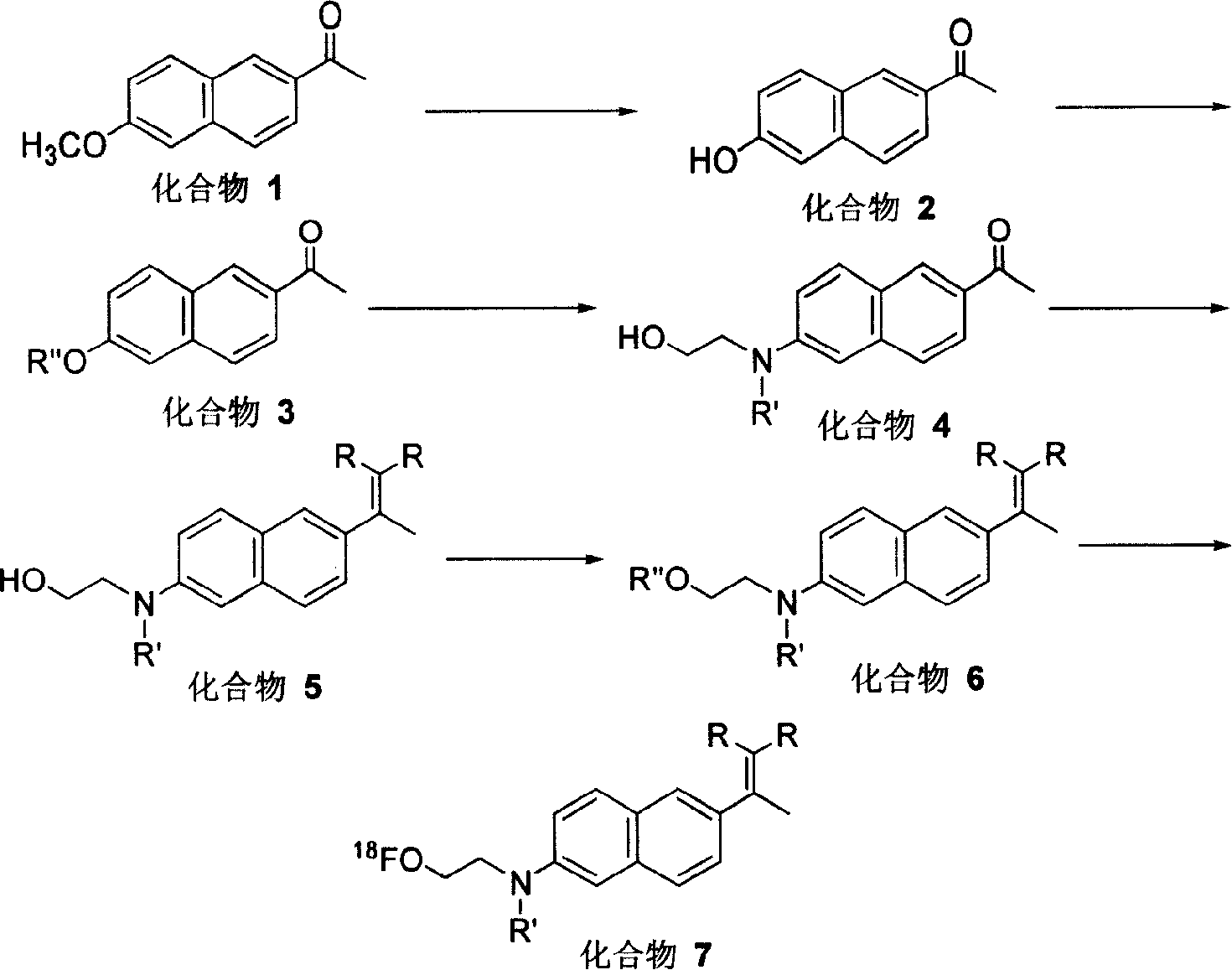
Synthesis method of image agent used for diagnosis of progeria dementia
A synthetic method, a technology for Alzheimer's disease, applied in chemical instruments and methods, drug combinations, preparation of organic compounds, etc., can solve problems such as limited effects and restrictions on the role of PET in Alzheimer's disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the synthesis of 6-hydroxyl-2-naphthylethanone (compound 2)
[0026] A solution dissolved in 80 mL of concentrated hydrochloric acid and 15 drops (about 0.75 mL) of triethylamine was heated to boiling. Add 1.00 g of 6-methoxy-2-naphthylethanone in CH 2 Cl 2 solution. After the reaction solution turned dark green, it was filtered through mineral wool to remove the black tar-like substance, washed with hot water, and solids precipitated after the solution cooled. The solid obtained by suction filtration was dissolved in 20 mL of ethyl acetate, washed with saturated sodium carbonate solution until neutral, and allowed to stand for liquid separation. The aqueous phase was extracted three times with 15 mL of ethyl acetate, the organic phases were combined, dried over anhydrous magnesium sulfate, and the solvent was evaporated to obtain 0.7311 g of a crude product with a yield of 78%.
Embodiment 2
[0027] Example 2: Synthesis of 2-acetyl-6-naphthyl-4'-methylbenzenesulfonate (compound 3a)
[0028]Will be dissolved with 35ml tetrahydrofuran, 0.35g (1.88mmol) 6-hydroxy-2-naphthyl ethyl ketone, 0.55g (2.88mmol) p-toluenesulfonyl chloride, 0.09g sodium hydroxide, 0.09g sodium bisulfite and 10 The three-necked flask dripped with triethylamine was heated to reflux, and the reaction was monitored by TLC (the developer was a mixture of acetone, dichloromethane and n-heptane, with a volume ratio of 3:6:3). After the reaction was completed, the resulting reaction mixture was dissolved in 30 ml of ethyl acetate, dried over anhydrous magnesium sulfate, filtered off insoluble matter, and the filtrate was volatilized and crystallized to obtain 0.45 g of crude product with a yield of 70.3%. The crude product was recrystallized from ethyl acetate, and the melting point of the pure product was 119-120°C.
Embodiment 3
[0029] Example 3: Synthesis of 2-acetyl-6-naphthyl acetate (compound 3b)
[0030] A solution containing 15ml of acetic anhydride, 0.60g (3.22mmol) of 6-hydroxy-2-naphthylethanone and 2 drops of triethylamine was heated to reflux for 2h. Filter out the insoluble matter, add 3 times of water to the filtrate, then add 2-3 drops of dilute HCl solution, stir, put in the refrigerator to freeze, and filter out the solid. The crude product was recrystallized from ethanol to obtain 0.60 g of needle crystals with a yield of 81.6%. The melting point is 85-86°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com