Tumour serum mark and its uses
A technology of serum markers and tumors, applied in the field of tumor serum markers and its application, can solve the problems that the sensitivity and specificity of tumor serum markers cannot meet clinical diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
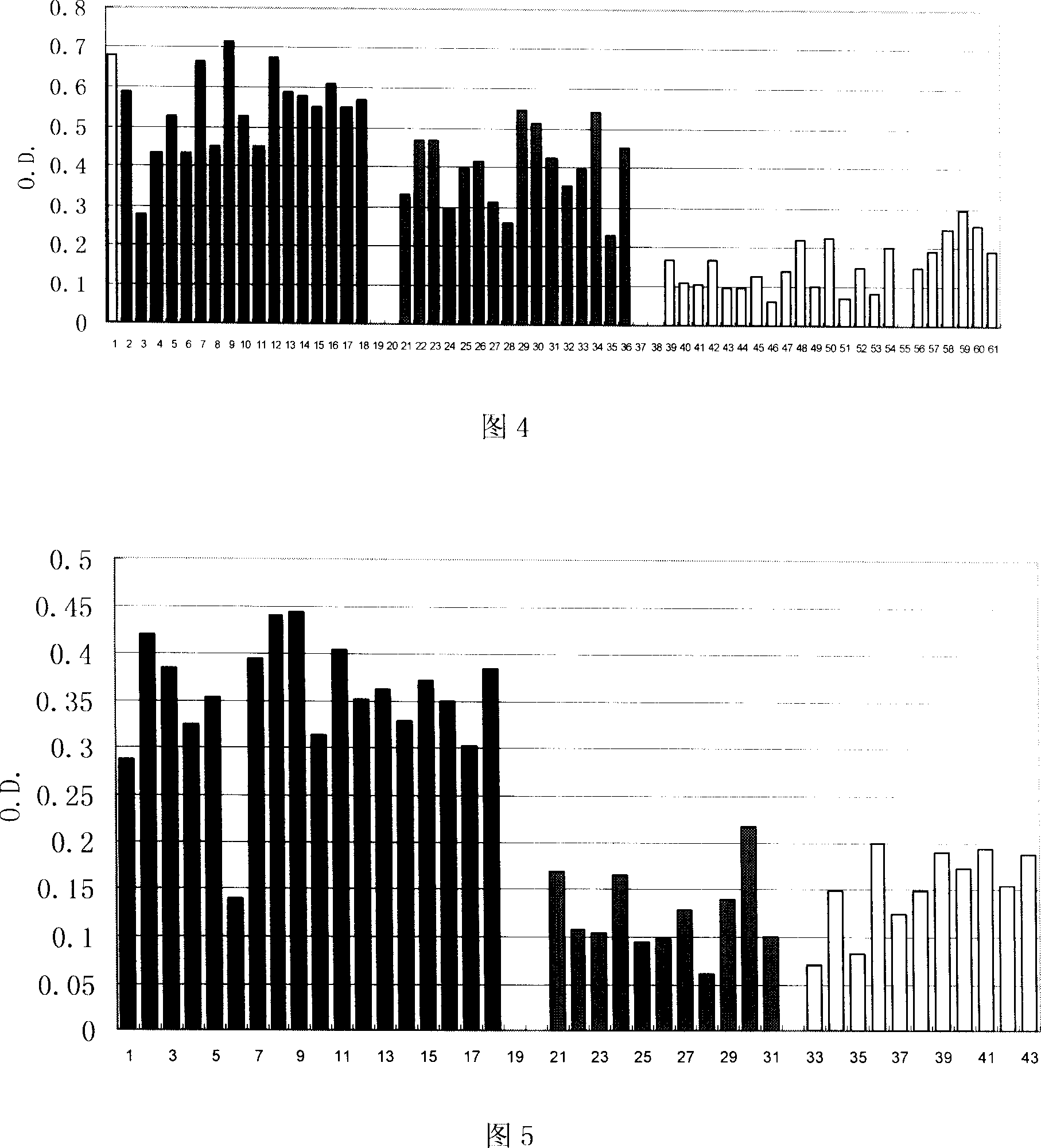
Image
Examples
Embodiment 1
[0029] Embodiment 1. Enzyme-linked immunosorbent assay (ELISA) measures the expression of PADI4 in serum
[0030] 1. Reagent Preparation
[0031] (1) 0.05M carbonate buffer (Carbonate-bicarbonate buffer) coating diluent, pH9.6:
[0032] Sodium carbonate (Na 2 CO 3 ) 1.59g, sodium bicarbonate (NaHCO 3 ) 2.93g, sodium azide (sodium azide) 0.2g, add water to 1000 ml, adjust the pH to 9.6.
[0033] (2) PBS buffer (pH7.4-7.6):
[0034] Sodium chloride (NaCl) 8g, anhydrous disodium hydrogen phosphate (NaCl) 2 HPO 4 ) 1.15g, potassium dihydrogen phosphate (KH 2 PO 4 ) 0.2g, potassium chloride (KCl) 0.2g, add water to 1 liter.
[0035] (3) Blocking solution:
[0036] Add 3 g of skimmed milk powder to 100 ml of PBS buffer (pH7.4) solution.
[0037] (4) PBS-Tween buffer (PBST) washing solution, pH7.4:
[0038] Add 0.5 ml of Tween-20 (Tween-20) to 1 liter of the above PBS buffer solution (pH7.4), add water to 1 liter, and adjust the pH to 7.4.
[0039] 2. The experiment was ca...
Embodiment 2
[0049] Embodiment 2: preparation ELISA (direct method) detects serum PADI4 kit
[0050] Serum PADI4 detection kit contains:
[0051] (1) ELISA plate (strip);
[0052] (2) 0.05M carbonate buffer sample coating diluent, pH9.6;
[0053] (3) Blocking solution: PBS buffer solution (pH7.4) containing 3% skimmed milk powder;
[0054] (4) Anti-PADI4 antibody, the antibody is routinely labeled with horseradish peroxidase;
[0055] (5) PBS antibody diluent: PBS buffer containing 2% BSA;
[0056] (6) Washing solution: PBS buffer containing 0.05% Tween-20 (PBST);
[0057] (7) Chromogen: 0.4% tetramethylbenzidine (TMB);
[0058] (8) Stop solution: 2% H 2 SO 4 .
[0059] (9) PADI4 recombinant protein or positive and negative control substances.
Embodiment 3
[0060] Embodiment 3: ELISA (direct method) detects the application of serum PADI4 kit
[0061] (1) Take the required amount of pre-coated plates (strips), dilute the serum to be tested 10-20 times with the sample diluent, add 100ul per well to the wells of the microplate, and place the microplate in a wet box at 4 degrees Celsius overnight.
[0062] (2) Discard the sample solution, add 200ul of PBST to each well, and then discard the PBST. Wash the plate 3 times with washing solution.
[0063] (3) Add 200ul of blocking solution to each well, and react the microplate plate in a humid box at room temperature for half an hour.
[0064] (4) Discard the blocking solution, and add 100ul of anti-PADI4 antibody to each well. The microtiter plate was reacted in a humid box at room temperature for 2 hours.
[0065] (5) Discard the primary antibody solution, and wash the microtiter plate three times with PBST.
[0066] (6) Add 100ul of chromogen to each well, and carry out chromogen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com