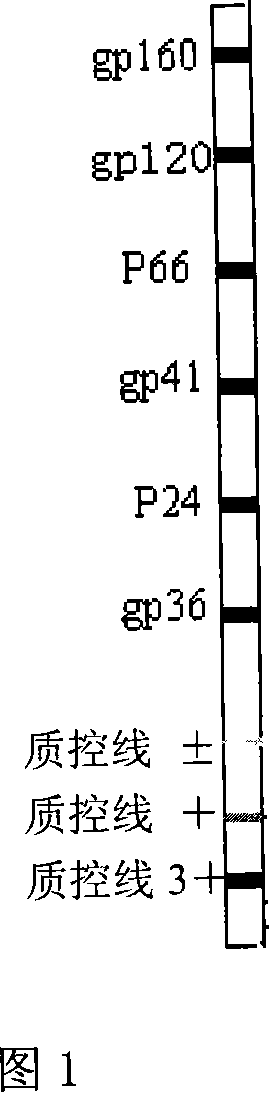Human immunodeficiency virus antibody confirmations reagent
A human immunodeficiency, antibody technology, applied in biological testing, material analysis by observing the impact on chemical indicators, material inspection products, etc., can solve the problems of complicated operation methods and long measurement time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] (1) Confirm the preparation and testing of test strips
[0123] with coating buffer. According to a certain concentration, the detection line and the quality control line are uniformly and neatly fixed on the mixed cellulose membrane with a flow test film scratcher.
[0124] Confirm the dryness of the test strip
[0125] After the coating was completed, it was placed on an ultra-clean workbench and air-dried at room temperature for 2 hours. Store in a dry environment at room temperature (16-25°C, relative temperature not higher than 45%).
[0126] semi-finished product inspection
[0127] The national reference product of HIV antibody confirmation reagent was used for verification.
[0128] The compliance rate of HIV antibody positive reference products is 10, and the required positive compliance rate is 10 / 10.
[0129] The HIV antibody negative reference product conformity rate was 12, and the required negative conformity rate was 12 / 12.
[0130] The coincidence ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com