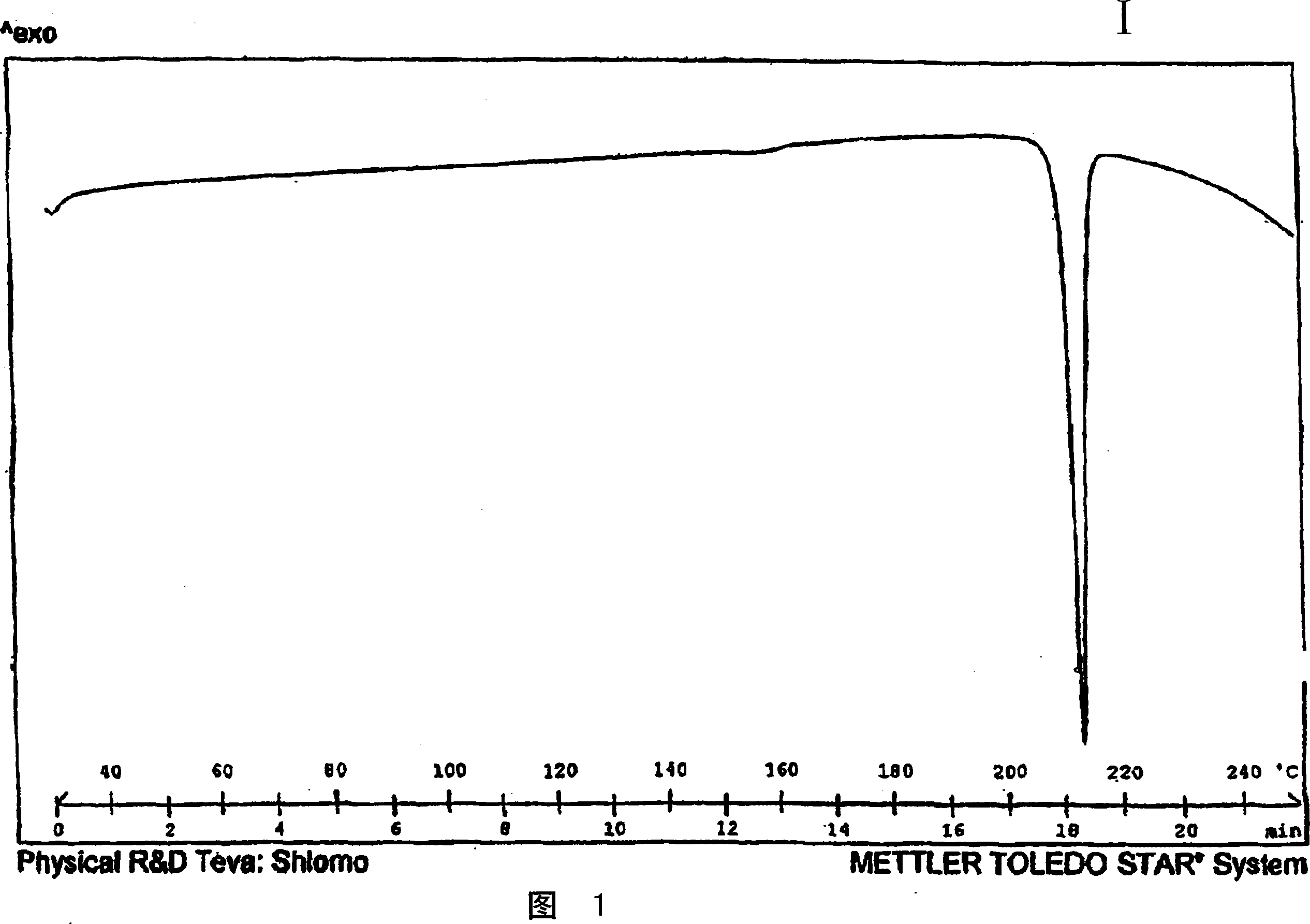
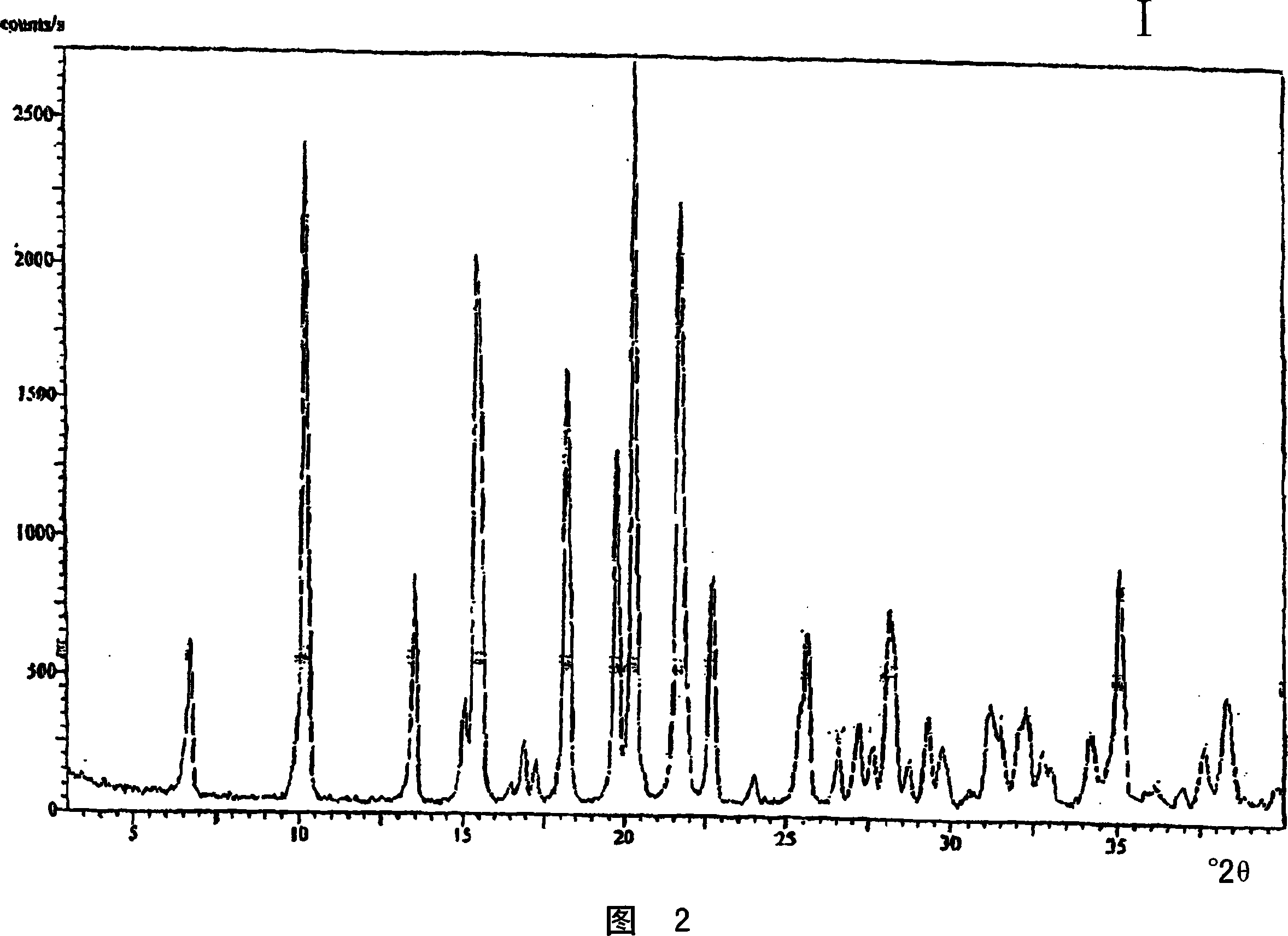
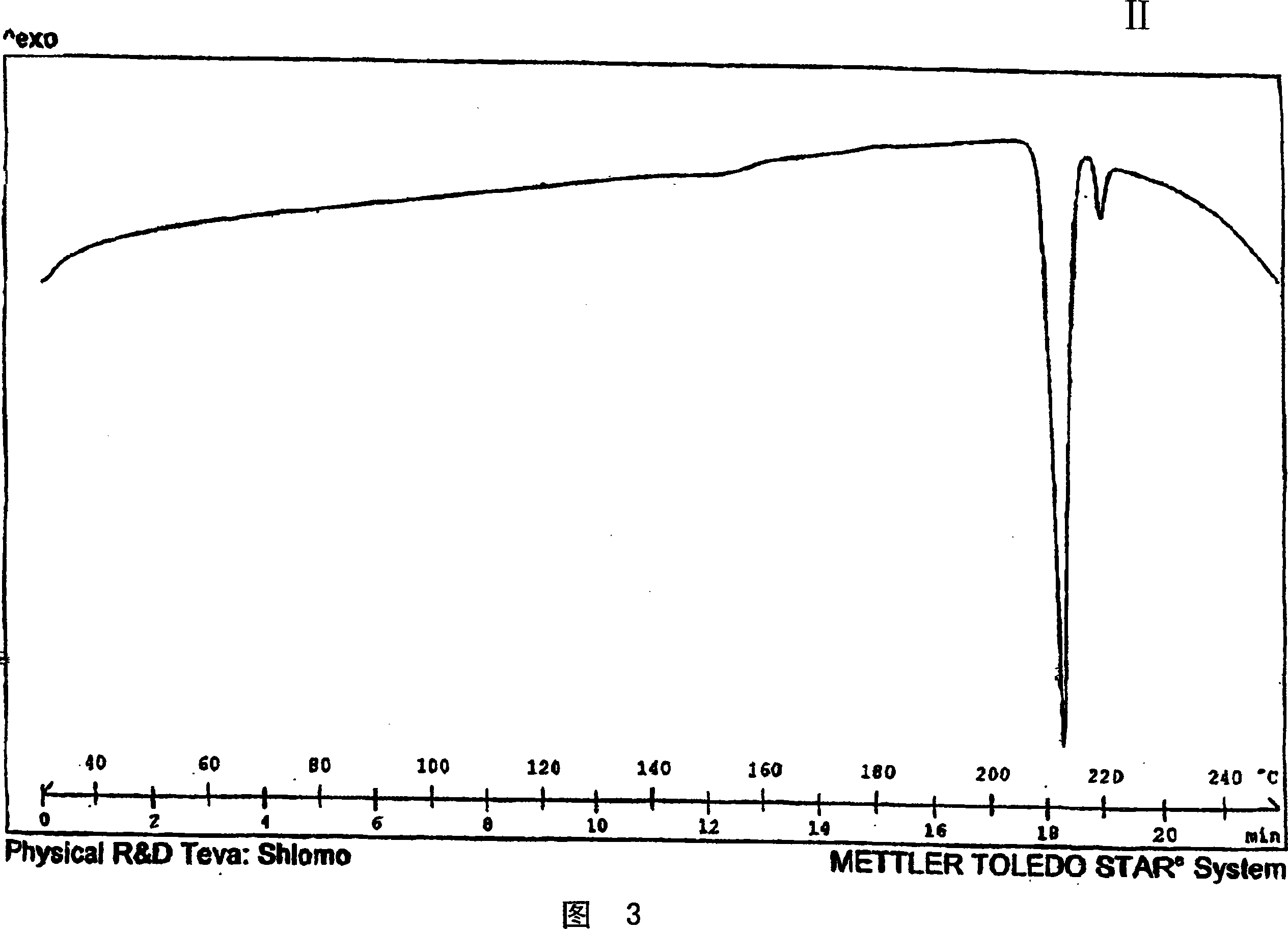
Crystalline venlafaxine base and novel polymorphs of venlafaxine hydrochlorid, processes for preparing thereof
A technology of venlafaxine hydrochloride and solvate, which is applied in the field of venlafaxine and can solve problems such as not describing venlafaxine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Aqueous 32% sodium hydroxide (10.0 g, 80.0 mmol) was added to a stirred solution of venlafaxine hydrochloride (20.0 g, 63.7 mmol) in water (100 mL) in an ice-water bath. The mixture was stirred in an ice / water bath for about 30 minutes and extracted with ethyl acetate (3 x 30ml). The combined organic layers were dried over anhydrous sodium sulfate, filtered and evaporated under reduced pressure at about 50-60°C (water bath). The residue was dissolved in boiling hexane (50ml) and cooled in a refrigerator (-18°C).
[0057] The resulting crystals were filtered off, washed with cold hexane (20 ml), and dried under reduced pressure to obtain 15.5 g (87.7%) of white venlafaxine crystals, whose purity was about 99.3% by HPLC, mp 78.3-79.5°C.
Embodiment 2
[0059] Preparation of crystalline venlafaxine free base from N,N-didesmethylvenlafaxine hydrochloride
[0060]
[0061] Aqueous 32% sodium hydroxide (2.75 g, 0.022 mol) was added to a stirred solution of N,N-didesmethylvenlafaxine hydrochloride (5.72 g, 0.02 mol) in water (13 ml) at room temperature. 88.5% aqueous formic acid (4.16 g, 0.08 mol) and 35.8% aqueous formaldehyde (3.7 g, 0.044 mol) were added to the emulsion. The resulting mixture was stirred at reflux for 8 hours, cooled to room temperature, adjusted to pH~11 with 32% aqueous sodium hydroxide solution, and extracted with heptane (100 ml).
[0062] The organic extract was washed with water (20ml), dried over sodium sulfate and evaporated to two volumes, and filtered to give crystalline venlafaxine base.
[0063] II) Venlafaxine Hydrochloride
[0064] The invention provides a method for purifying venlafaxine hydrochloride, which comprises alkalizing venlafaxine hydrochloride.
[0065] The invention provide...
Embodiment 3
[0119] Preparation of Type III and Type I with Solvents / Antisolvents
[0120] Ratio: 0.7ml water: 9.7ml acetone: 3 grams venlafaxine hydrochloride
[0121] Under reflux conditions, venlafaxine hydrochloride was dissolved in water and acetone was added. The resulting suspension was refluxed for a further 10 minutes and left overnight at room temperature. Afterwards, the suspension was filtered and washed with about 2 ml of the same solvent mixture. The resulting solid crystallized out as type III. Form I was obtained by further drying under reduced pressure (~10 mbar) for about 45 minutes (or more) in a rotary evaporator at about 60°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com