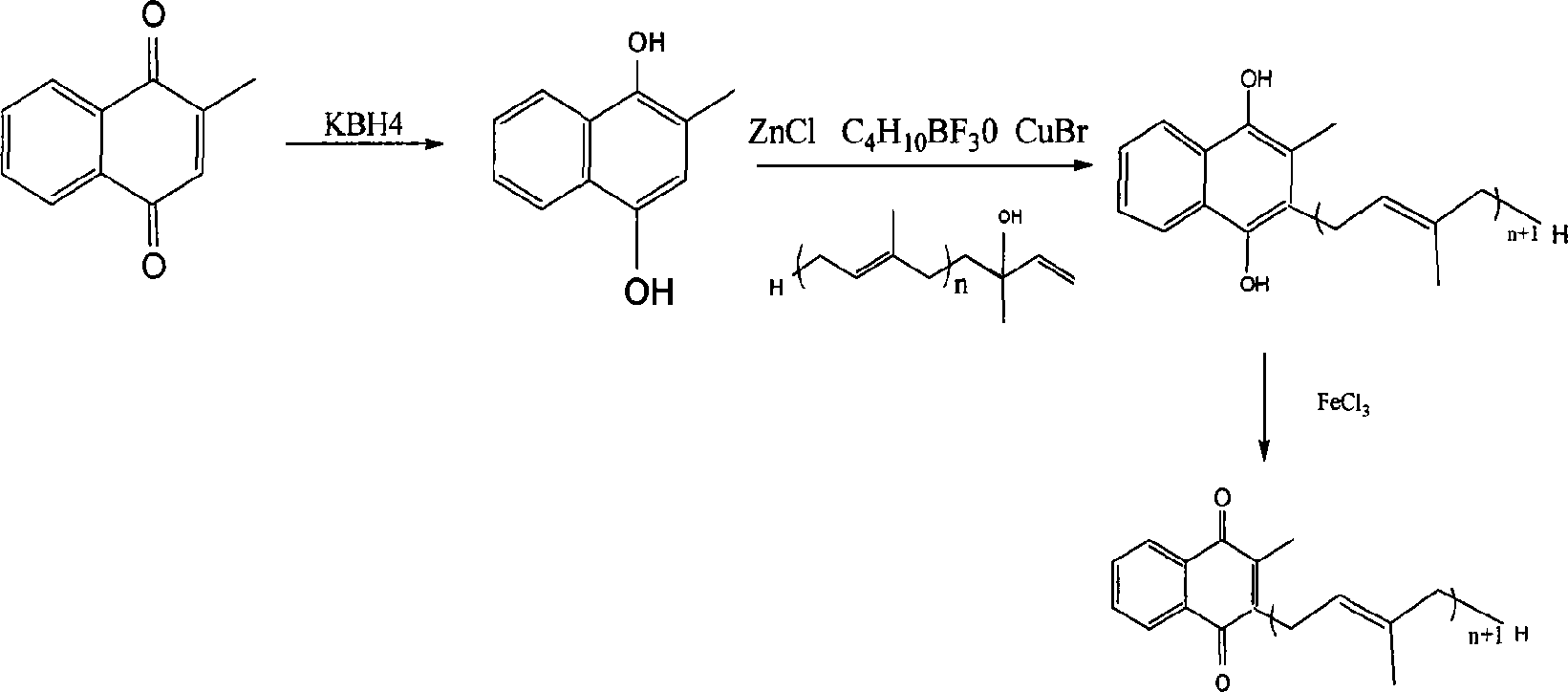
Method for synthesizing producets in vitamin K2 series
A synthetic method and technology of vitamins, applied in chemical instruments and methods, preparation of organic compounds, chemical recovery, etc., can solve the problems of low yield and many side reactions, and achieve the goal of reducing environmental pollution, easy operation and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0034] (1) Preparation of 2-methyl-1,4-naphthoquinone hydroquinone: 516g (3mol) 2-methyl-1,4-naphthoquinone is placed in a 5000mL three-necked bottle, equipped with a mechanical stirrer, and 2000mL of water is added and 2000mL ethanol, stirred, and added 107.9g (2mol) KBH in portions 4 , control the reaction temperature not to exceed 50°C, and after the addition is completed, seal and stir until the reaction solution is light green, under the protection of nitrogen flow, use dilute hydrochloric acid to slowly neutralize to pH 4, extract three times with ethyl acetate, combine ethyl acetate, Anhydrous Na 2 SO 4 Dry, recover about 70% ethyl acetate, cool to room temperature, freeze and crystallize in the refrigerator for 12 hours, filter, recover part of the ethyl acetate from the filtrate, freeze and crystallize, combine the obtained crystals twice, heat and dissolve with ethyl acetate, Freeze and recrystallize in the refrigerator, filter, and drain the crystals to obtain 472...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com