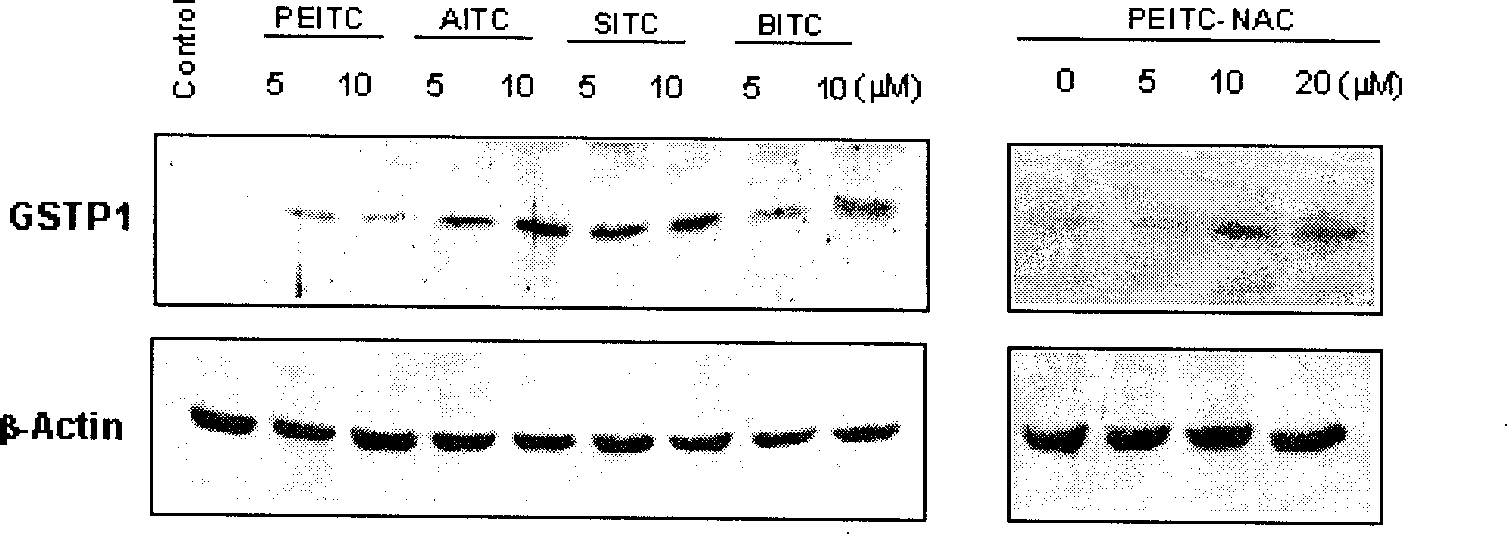
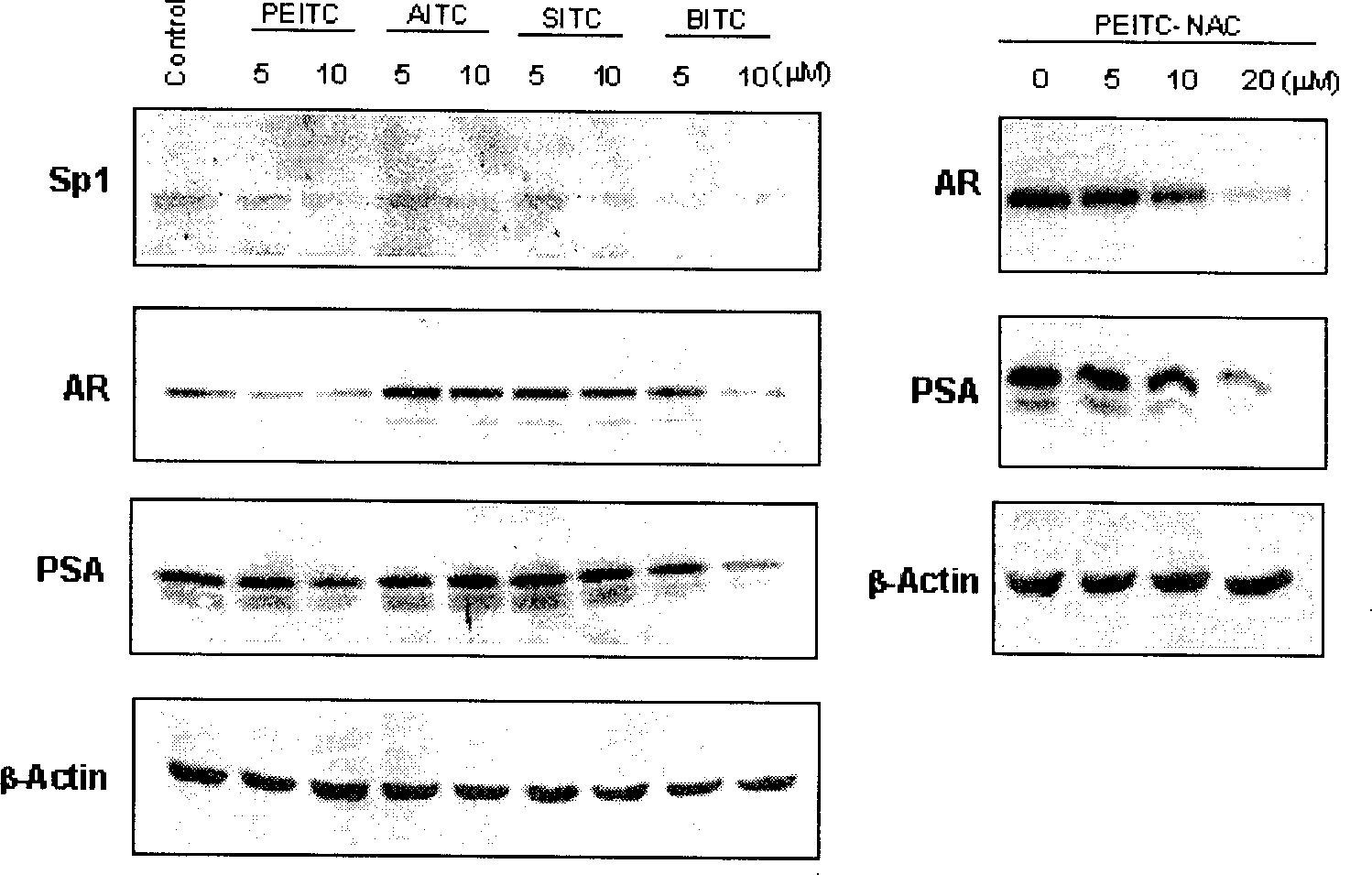
Application of compounds in isorhodanic ester classes for treating diseases of prostate and skin cancer
A technology of ester compound and isothiocyanate, which is applied in the application field of isothiocyanate compound in prostate diseases and skin cancer, can solve the problems of affecting stability, poor stability and the like, and achieves increased removal of harmful substances The ability, treatment and or prevention of prostate disease and other tissue and organ inflammation, the effect of preventing prostate disease and other tissue and organ inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Embodiment 1: the preparation of phenylethyl isothiocyanate
[0090] Instruments and reagents:
[0091] 1 H-NMR spectrum was measured by BruckerAV-300 nuclear magnetic resonance instrument, internal standard TMS, and the solvent was CDCl unless otherwise specified. 3 ; MS was determined with a Nicolet FTMS-2000 mass spectrometer; elemental analysis was determined with an Elementar Vario EL III instrument.
[0092] Thin layer chromatography (TLC) using silica gel GF 254 (Produced by Qingdao Ocean Chemical Factory) self-made; all reagents are commercially available chemically pure or analytically pure products, and are used directly without treatment.
Embodiment 1-1
[0093] Example 1-1: Extracting phenylethyl isothiocyanate from natural plants
[0094] a. Cut watercress into pieces, add water, and place it at room temperature for several days. Under the action of enzymes, phenylethyl isothiocyanate is decomposed, extracted with a water-immiscible solvent such as n-hexane, and then vacuum evaporated to remove the solvent , and then distilled under reduced pressure to obtain the product, the product can obtain higher yield and purity [6] (For specific methods, see: Ribnicky David M, Poulev Alexander A et al. 2000).
[0095] b. According to the above method, phenylethyl isothiocyanate can also be obtained from the winter unicorn; after soaking the root pieces of mignonette in water, and then steam distillation, the above-mentioned phenylethyl isothiocyanate can be obtained.
Embodiment 1-2
[0096] Embodiment 1-2: Synthesis of phenylethyl isothiocyanate by chemical method
[0097] Add 15 mL of CH to a 50 mL round bottom flask 2 Cl 2 and thiophosgene 3mL (40mmol), stirred, cooled to 0°C, and slowly added dropwise a mixture of equivalent triethylamine (4.04g, 40mmol) with a constant pressure dropping funnel (the addition process exothermic, the temperature should not exceed 15°C). After dripping, react at room temperature for 5-6 hours, after detecting the disappearance of phenethylamine by TLC, add 10 mL of water to quench the reaction. Add 5mLCH 2 Cl 2 , separated the organic phase with a separatory funnel, washed the organic phase twice with water (15 mL×2), dried the organic phase over anhydrous sodium sulfate, filtered, and concentrated the organic phase to dryness. The residual liquid used petroleum ether (boiling range 60-90° C.) as the eluent, was eluted and purified through a silica gel column, concentrated, and then vacuum-distilled to obtain 4.9 g of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com