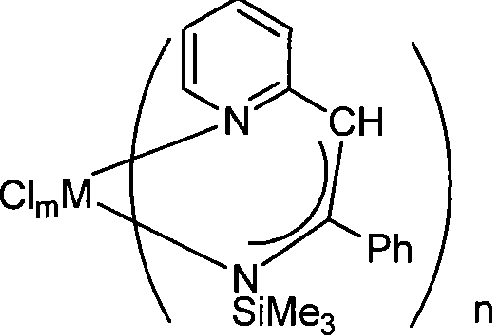
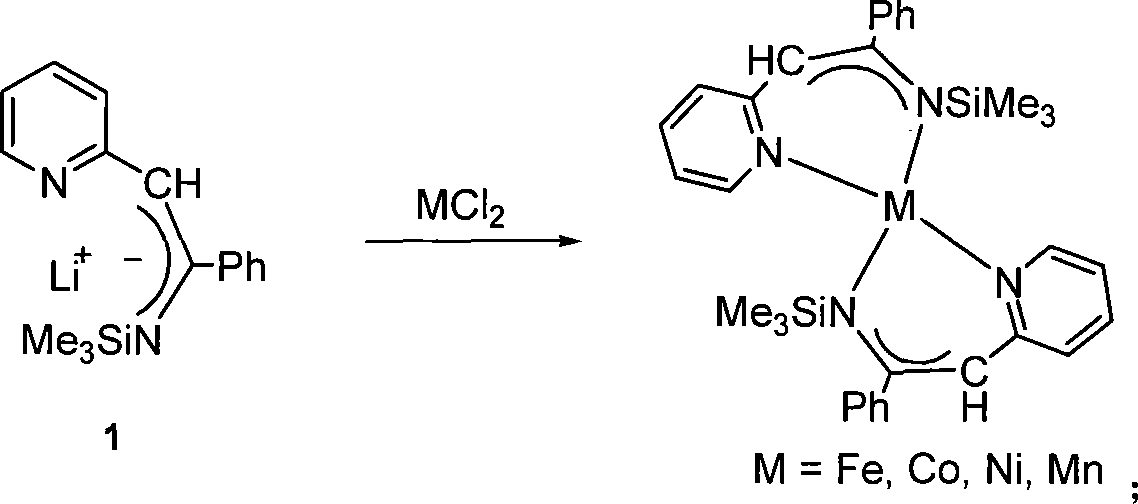
Metal complex using pyridine as matrix and synthetic method thereof
A technology of metal complexes and precursors, applied in the field of metal complexes and their synthesis, can solve the problems of low yield, high cost, complicated catalyst preparation and the like, and achieve the effects of high product yield and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Co[C 5 h 4 NCHC(Ph)NSiMe 3 ] 2 preparation of
[0025] Take 2-picoline (2.569g, 27.59mmol) in a Schlenk bottle, add about 30ml of ether to dissolve it. At 0°C, an equimolar amount of n-butyllithium (9.5ml, 27.59mmol) was added thereto, the color of the solution turned red, and after returning to room temperature, stirred for 4 hours to obtain compound (C 5 h 4 NCH 2 ) Li. At 0°C, continue to add an equimolar amount of trimethylchlorosilane (3.49ml, 27.59mmol) to the solution, and after returning to room temperature, stir for 4 hours to obtain a yellow turbid solution, which is left to stand and filtered to obtain compound C 5 h 4 NCH 2 SiMe 3 light yellow solution. The calculated yield is 93%.
[0026] Under ice-bath cooling conditions, take n-butyllithium (8.8ml, 25.66mmol), add the C obtained above 5 h 4 NCH 2 SiMe 3 In the solution, the solution turned dark red, and after returning to room temperature, stirred for 4 hours to obtain the comp...
Embodiment 2
[0036] Embodiment 2: [C 5 h 4 NCHC(Ph)NSiMe 3 ]SnCl 3 preparation of
[0037] Take η 3 - Azaallyl ligand compound (1) (1.234g, 4.5mmol), add about 10ml of ether solvent, slowly add SnCl dropwise to it at -78°C 4 (0.53ml, 4.5mmol), the solution turned into a yellow turbid liquid, and after returning to room temperature for 12 hours, the solvent was removed under reduced pressure, and CH 2 Cl 2 Extract, filter, concentrate the filtrate, put it at room temperature to crystallize, and after 3 days, a yellow regular crystal compound [C 5 h 4 NCHC(Ph)NSiMe 3 ]SnCl 3 , (1.728g, 78%). 1 H NMR (300Hz, 298K, CDCl 3 ): δ (ppm) 0.14 (s, 9H, SiMe 3 ), 5.97(s, 1H, CH), 7.20-7.86(8H, Py and Ph), 8.96(d, 1H, Py). 13 C NMR (75MHz, 298K, CDCl 3 ): δ (ppm) 3.14 (SiMe 3 ), 106.63(CH), 120.48-161.29(Py, C=N andPh). Its molecular structure is as follows:
[0038]
[0039] [C 5 h 4 NCHC(Ph)NSiMe 3 ]SnCl 3 molecular structure
[0040] [C 5 h 4 NCHC(Ph)NSiMe 3 ]SnCl 3 Part ...
Embodiment 3
[0045] Embodiment 3: [C 5 h 4 NCHC(Ph)NSiMe 3 ]GeCl 3 preparation of
[0046] Take crystal compound 1 (0.745g, 2.75mmol), add about 10mlEt 2 O diethyl ether solvent, slowly drop GeCl into it at -78°C 4 (0.31ml, 2.75mmol), the solution turned into an orange turbid liquid, after returning to room temperature, reacted for 12 hours, the solvent was removed under reduced pressure, and CH 2 Cl 2 Extract, filter, concentrate the filtrate, put it at room temperature to crystallize, and after 3 days, yellow blocky crystals appear [C 5 h 4 NCHC(Ph)NSiMe 3 ]GeCl 3 , (0.994g, 81%). 1 H NMR (300Hz, 298K, CDCl 3 ): δ (ppm) 0.053-0.22 (SiMe 3 ), 5.70 (CH), 7.10-7.77 (Py and Ph), 8.79 (Py).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com