Method of preparing polyhydroxy annular nitrone
A polyhydroxyl and cyclic technology, applied in organic chemistry and other directions, can solve the problems of limited research and new drug development process, low yield of polyhydroxyl cyclic nitrones, and inability to prepare in large quantities, and achieve efficient preparation and preparation methods. Simple, high-yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
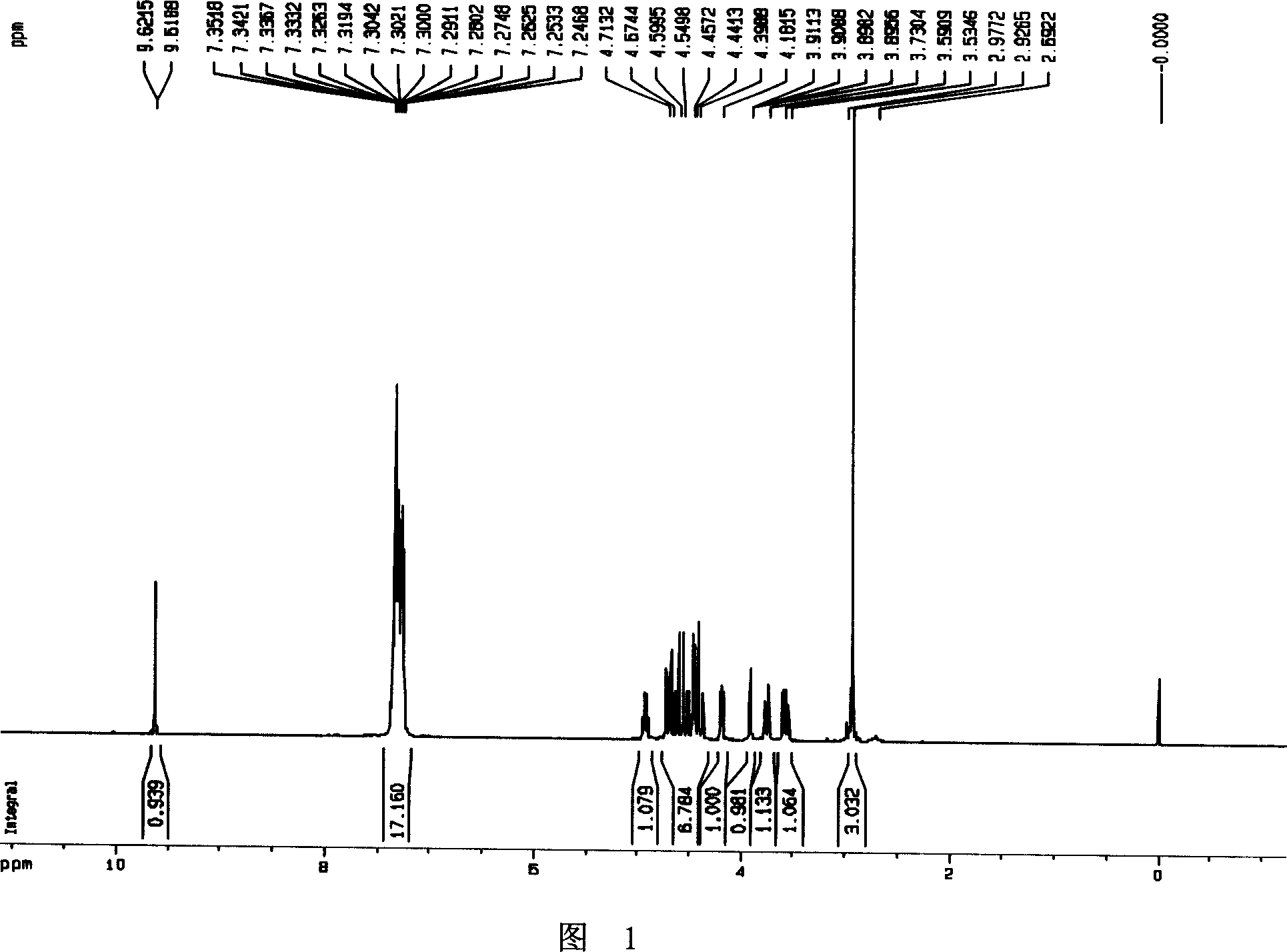
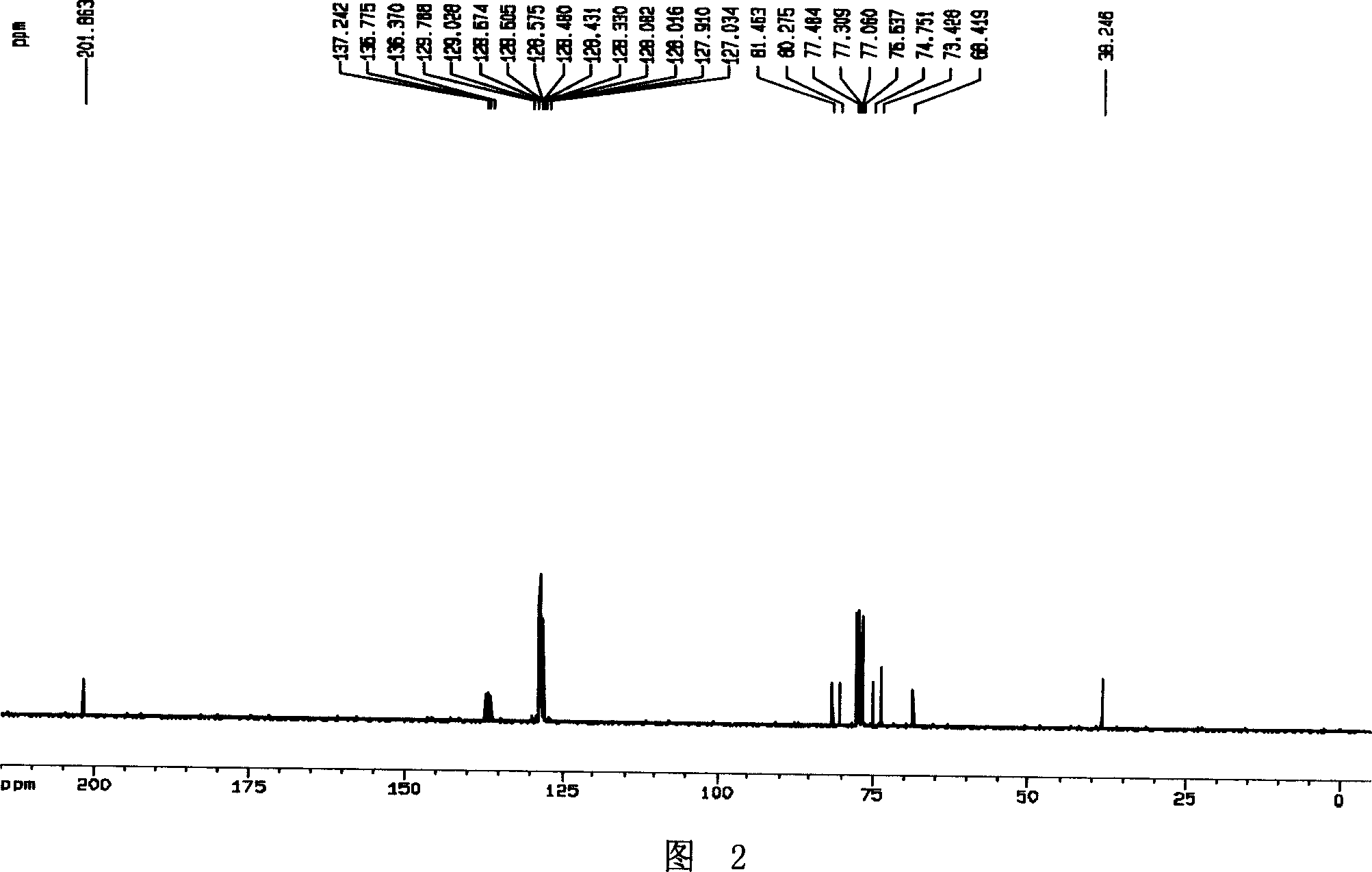
[0029] Example 1, Preparation of polyhydroxy cyclic nitrone (2S, 3S, 4S)-2-benzyloxymethyl-3,4-dibenzyloxy-3,4-dihydro-1-oxypyrrole I
[0030]
[0031] According to literature methods [(a) Barker, R.; Fletcher, H.G.J.Org.Chem. 1961, 26, 4605-4609. (b) Tejima, S.; Fletcher, H.G.J.Org.Chem. ] Preparation of the hemiacetal 2,3,5-O-tribenzyl-D-xylfuranose II from D-xylose. Pyridine (30mL, 0.37mol) was added to the dichloromethane (100mL) solution of the hemiacetal crude product (calculated according to 0.2mol), and O-methylhydroxylamine hydrochloride (20.88g, 0.25mol) was added thereto, at room temperature After stirring for 12 hours, the solvent was evaporated to dryness, then ethyl acetate and hydrochloric acid (1N, 30mL) were added to the concentrated solution, and after extraction and separation, the organic phases were combined, dried, and concentrated to obtain a yellow oil (2S, 3S, 4R )-2,3,5-tribenzyloxy-4-hydroxyl-1-pentanal methyl oxime ether III, directly put into t...
Embodiment 2
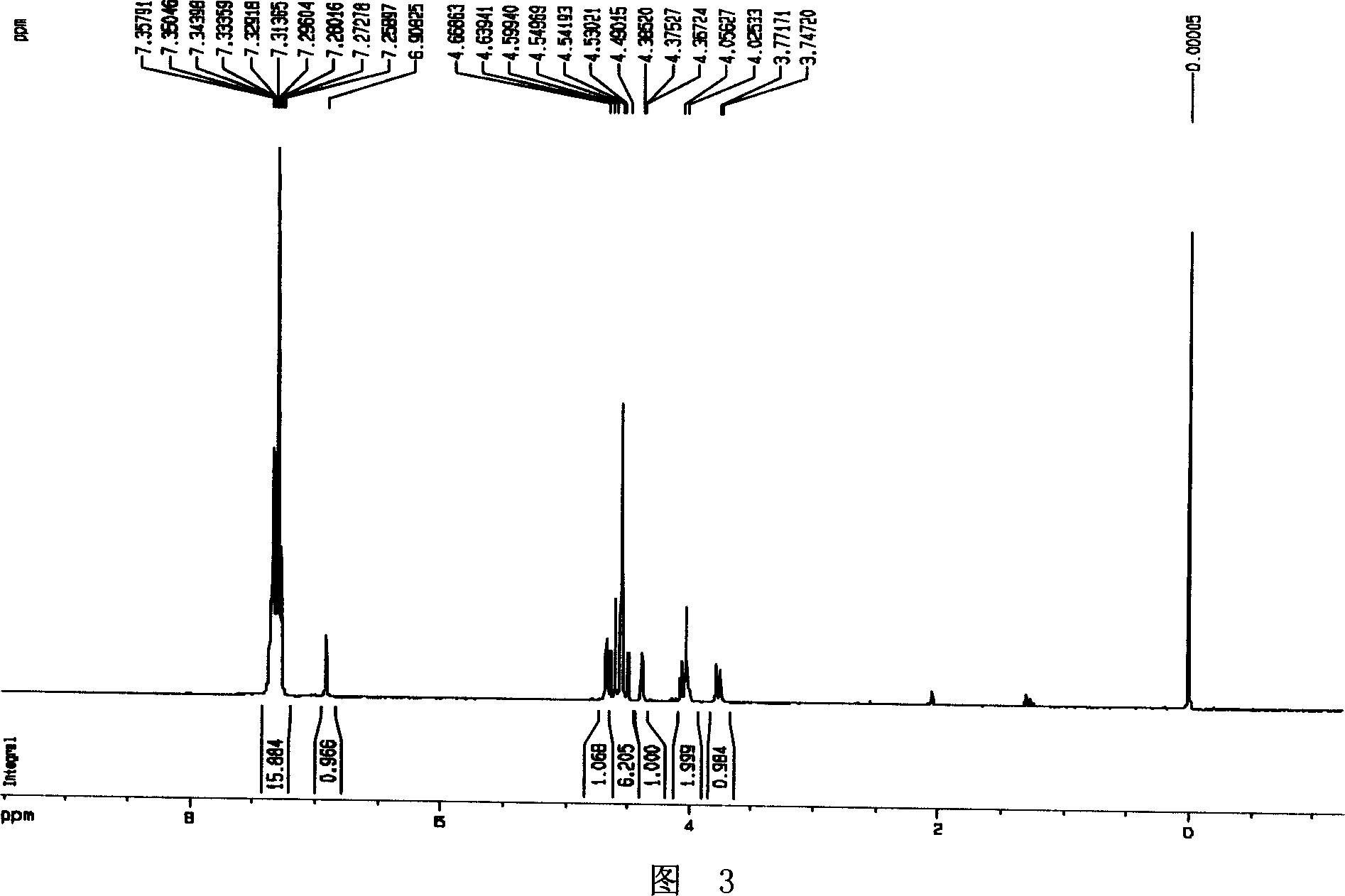
[0042] Example 2, Preparation of polyhydroxy cyclic nitrone (2R, 3R, 4S)-2-benzyloxymethyl-3,4-dibenzyloxy-3,4-dihydro-1-oxypyrrole I
[0043]
[0044] According to literature methods [(a) Barker, R.; Fletcher, H.G.J.Org.Chem. 1961, 26, 4605-4609. (b) Tejima, S.; Fletcher, H.G.J.Org.Chem. ], Preparation of the hemiacetal 2,3,5-O-tribenzyl-D-arabinofuranose II from D-arabinose. Pyridine (2.7 mL, 34.2 mmol) was added to a solution of the hemiacetal (8.40 g, 11.4 mmol) in dichloromethane (20 mL), and O-methylhydroxylamine hydrochloride (1.91 g, 22.8 mmol) was added thereto, After stirring at room temperature for 24 hours, the solvent was evaporated to dryness, then ethyl acetate and hydrochloric acid (1N, 30mL) were added to the concentrated solution, after extraction and separation, the organic phases were combined, dried and concentrated, and the obtained crude product was purified by column chromatography to obtain Pale yellow oil (2R,3R,4R)-2,3,5-tribenzyloxy-4-hydroxy-1-...
Embodiment 3
[0051] Example 3: Addition reaction of nitrone and phenylmagnesium bromide to obtain (2S, 3S, 4S, 5S)-2-(benzyloxymethyl)-3,4-dibenzyloxy-5-phenyl- N-Hydroxypyrrolidine VI
[0052]
[0053] Under nitrogen protection conditions, bromobenzene (0.26mL, 2.5mmol) was added to the suspension of magnesium strips (60mg, 2.5mmol) and freshly distilled tetrahydrofuran (5mL), and heated to reflux for 3 hours to prepare a tetrahydrofuran solution of phenylmagnesium bromide . In an ice bath, add nitrone (2R, 3R, 4R)-2-benzyloxymethyl-3,4-dibenzyloxy-3,4-dihydro-1-oxopyrrole containing vigorously stirring (200mg, 0.5mmol) tetrahydrofuran solution was added dropwise to the Grignard reagent prepared by the above method, and stirring was continued for half an hour after the addition was completed. Then add saturated aqueous ammonium chloride solution to quench the reaction, add ethyl acetate for extraction, and combine the organic phases. The solvent was spin-dried under vacuum, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com