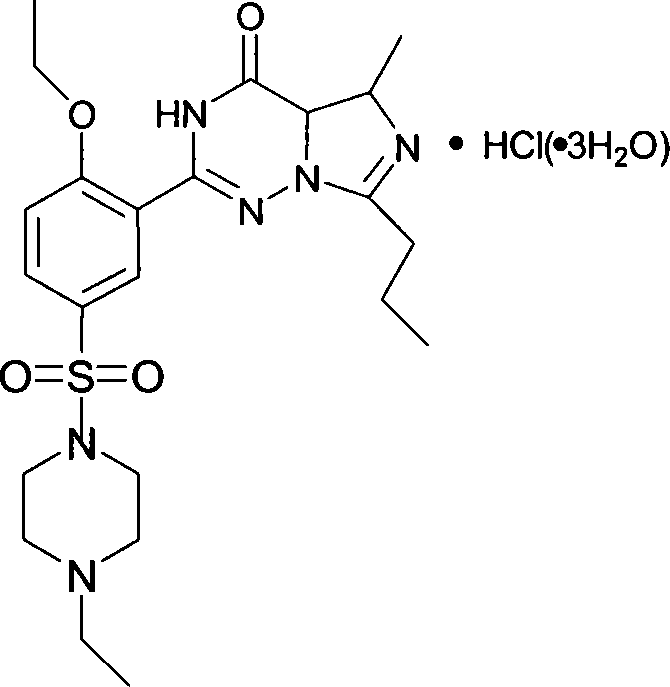
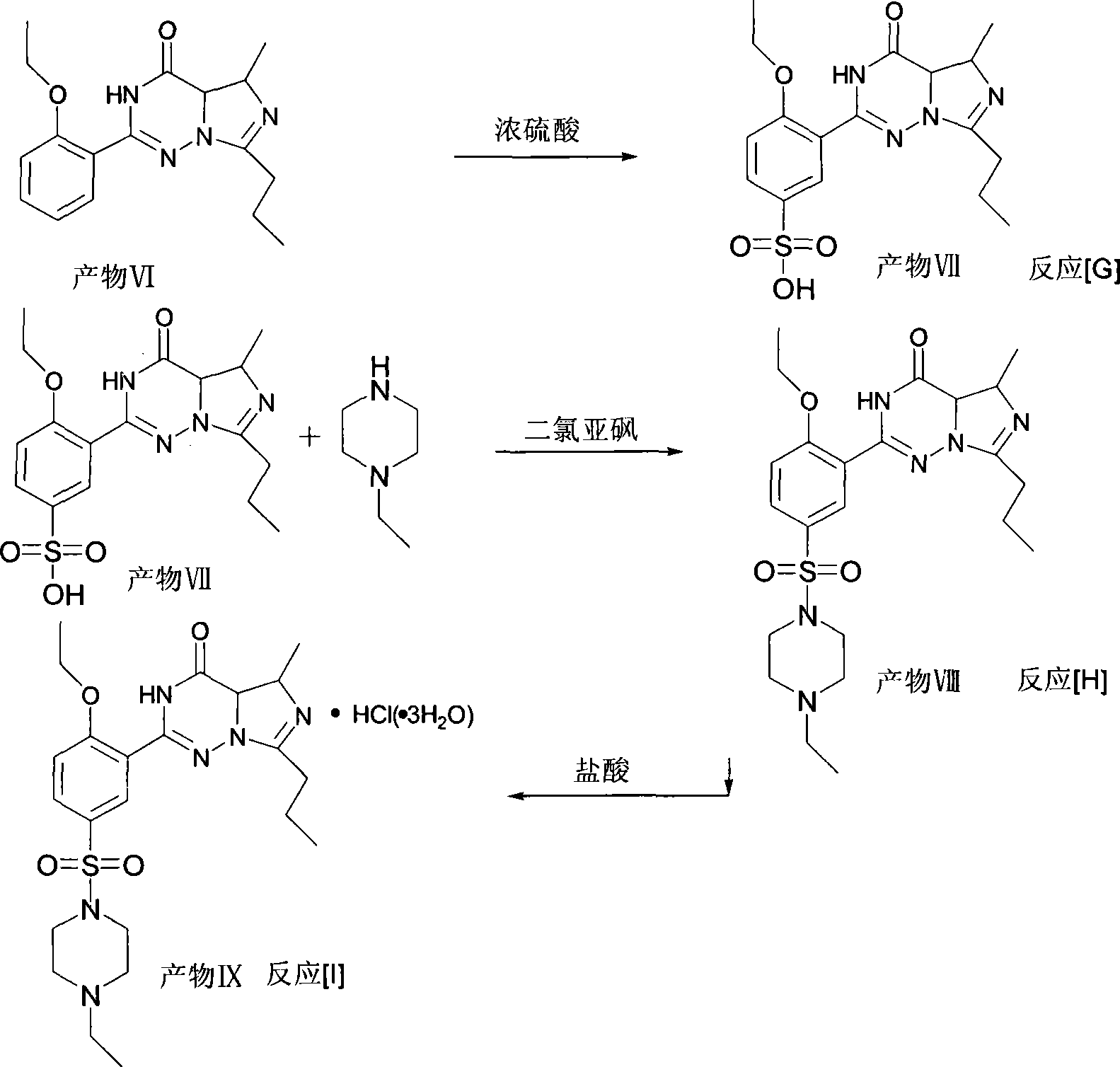
Method for synthesizing Vardenafil hydrochloric acid
A technique of vardenafil hydrochloride and a synthetic method, which is applied in the field of organic synthesis, can solve the problems affecting the state and purity of product VI, reduce the yield of product VII, and the product is not easy to precipitate, so as to achieve easy operation, reduce production cost, and react easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045](1) Add 10.04g of D, L-alanine to a mixed solvent made of 56ml of acetone and 92ml of water, then add 6.24g of sodium hydroxide, then add dropwise 18ml of 9mol / L sodium hydroxide solution and 13ml of For n-butyryl chloride, maintain the reaction at 0-8°C for 1.5 hours, adjust the reaction solution to pH=1 with hydrochloric acid, extract with ethyl acetate, dry over anhydrous sodium sulfate, and distill under reduced pressure to obtain 2-butyrylaminopropionic acid (product 1 ) 13.66g, its yield is 76.2%.
[0046] (2) Dissolve 2.0 g of the product obtained in step (1) in 13 ml of tetrahydrofuran, add 0.06 g of 4-N, N lutidine, then add 3.2 g of pyridine, stir, and slowly add oxalyl chloride mono Ethyl ester was refluxed in an oil bath for 3 hours, filtered, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, and distilled under reduced pressure to obtain 3-butyrylamino-2-oxo-butyric acid ethyl ester (product 2) 1.90 g, the yield...
Embodiment 2
[0053] In step (1), the mixed solvent is 56ml of acetone and 56ml of water, and the weight of the obtained product 1 is 12.37g, and its yield is 69%. Other steps are with embodiment 1.
Embodiment 3
[0055] In step (1), the reaction solution was adjusted to pH=3 with hydrochloric acid, and the weight of product 1 was 11.86 g, and the yield was 66.2%. Other steps are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com