Gitamycin slow-release micro-ball preparation and its preparing process
A technology of kitasamycin and slow-release microspheres, which is applied in the improvement of the dosage form of kitasamycin premix and the invention field of the preparation process of kitasamycin slow-release microspheres, which can solve the problem of large material consumption, high cost, and large amount of dust, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
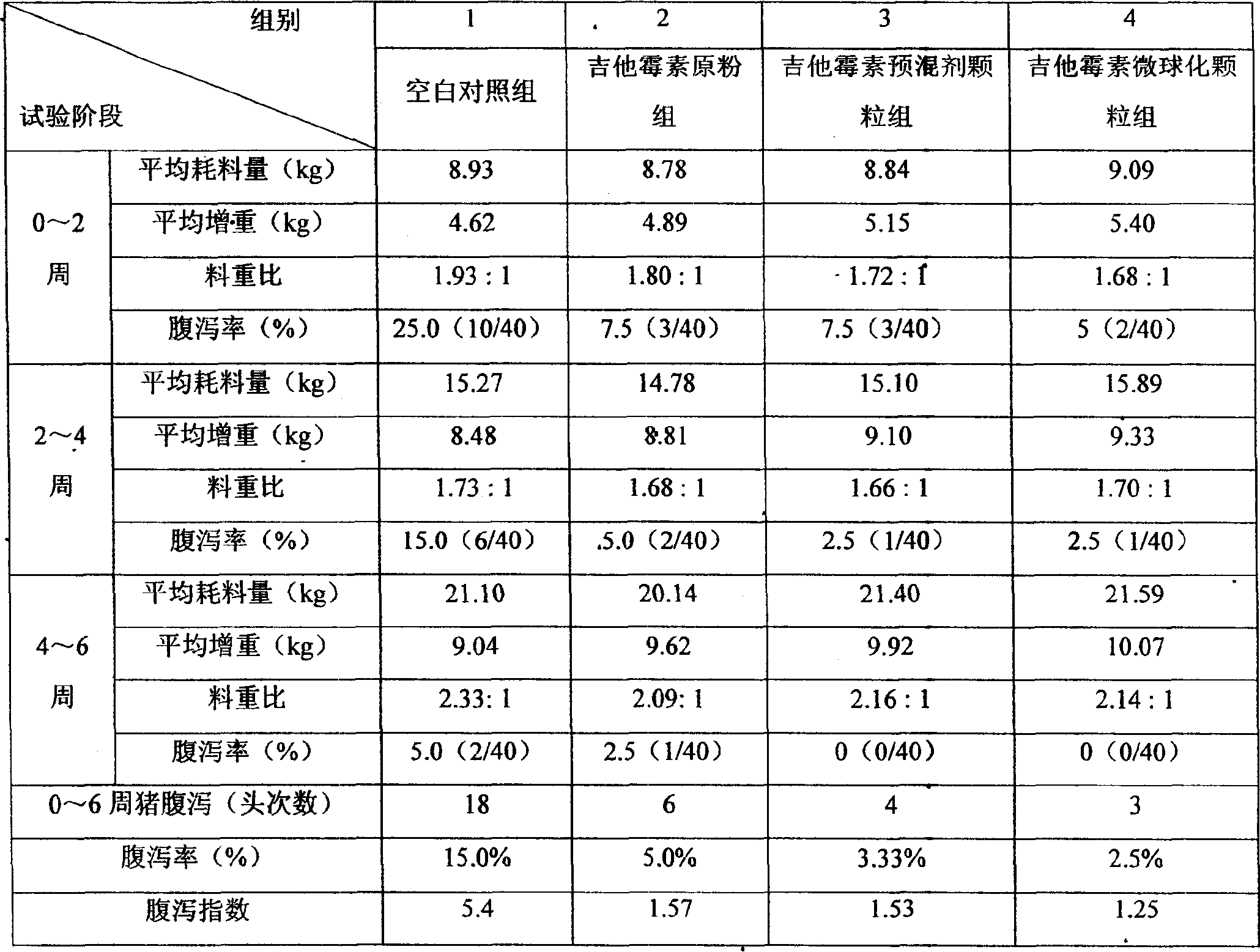
[0037] Select 225kg of stearic acid into the chemical material tank, heat to 80°C, and stir continuously until all the auxiliary materials are melted; pump the melted stearic acid from the chemical material tank into the compounding tank, and at the same time, accurately weigh the potency of 1636μ / mg Put 100kg of kitasarmycin into the batching tank, shear and stir for 10 minutes, during which the temperature should be controlled at 80°C; import the prepared material into the storage tank, and heat the temperature to 80°C-100°C; start the conveying spray device, The materials in the storage tank are sprayed and granulated; the finished product particles are collected by sieving with a 24-mesh vibrating sieve. Finally, 313kg of the finished kitasarmycin microsphere preparation was obtained, the particle size was 250 μm-700 μm, and the yield could reach 96%.
example 2
[0039] Select 175kg of stearic acid into the chemical material tank, heat to 80°C, and stir continuously until all the auxiliary materials are melted; pump the melted stearic acid from the chemical material tank into the compounding tank, and at the same time, accurately weigh the potency of 1617μ / mg Put 80kg of kitasarmycin into the batching tank, shear and stir for 8 minutes, during which the temperature should be controlled at 80°C; import the prepared material into the storage tank, and heat the temperature to 80°C-100°C; start the feeding spray device, The materials in the storage tank are granulated through spray freezing equipment; the finished product granules are collected by sieving with a 24-60 mesh vibrating sieve. Finally, 245 kg of the finished kitasarmycin microsphere preparation is obtained, the particle size is 250 μm-700 μm, and the yield can reach 96%.
example 3
[0041] Select 220kg of calcium stearate monoglyceride into the chemical material tank, heat to 80°C, and stir continuously until all the auxiliary materials are melted; pump the melted calcium stearate monoglyceride from the chemical material tank into the batching tank, and simultaneously Weigh 100kg of kitasarmycin with a potency of 1617μ / mg into the batching tank, shear and stir for 8 minutes, during which the temperature should be controlled at 80°C; import the prepared materials into the storage tank, and heat the temperature to 80°C-100°C ℃; start the feeding and spraying device, and granulate the materials in the storage tank through the sprayer; pass the finished product granules through the cooling system and sieve through the 24-60 mesh vibrating sieve to collect. Finally, 303.5 kg of finished kitasarmycin microsphere preparations (50%) were obtained, the particle size was 250 μm-700 μm, and the yield could reach 95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com