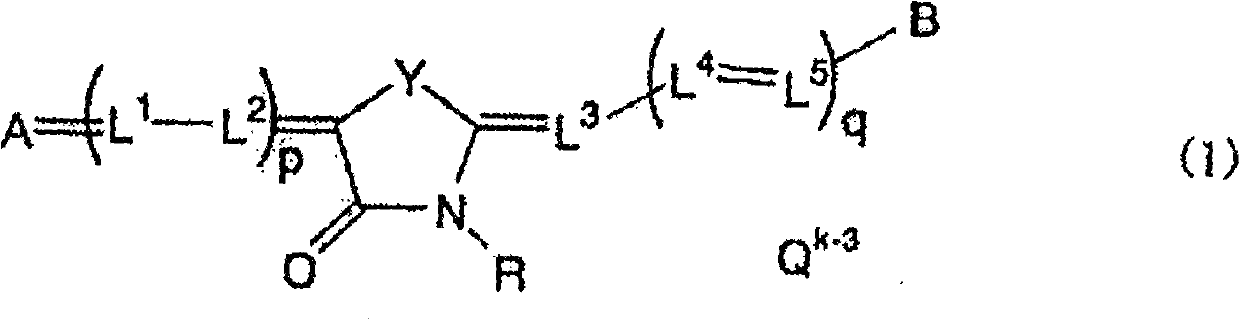
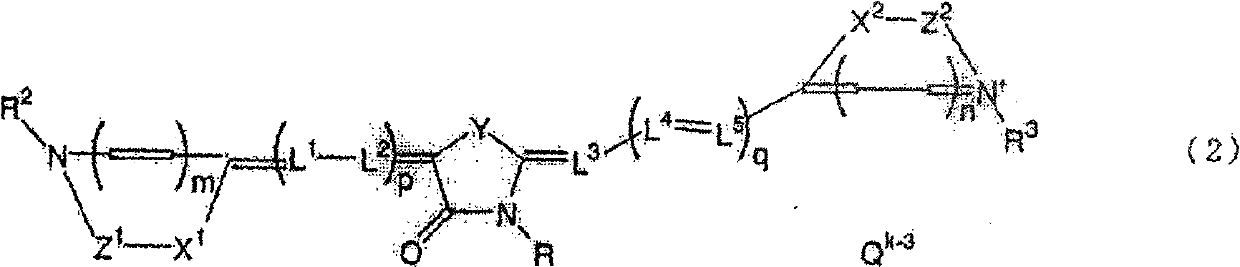
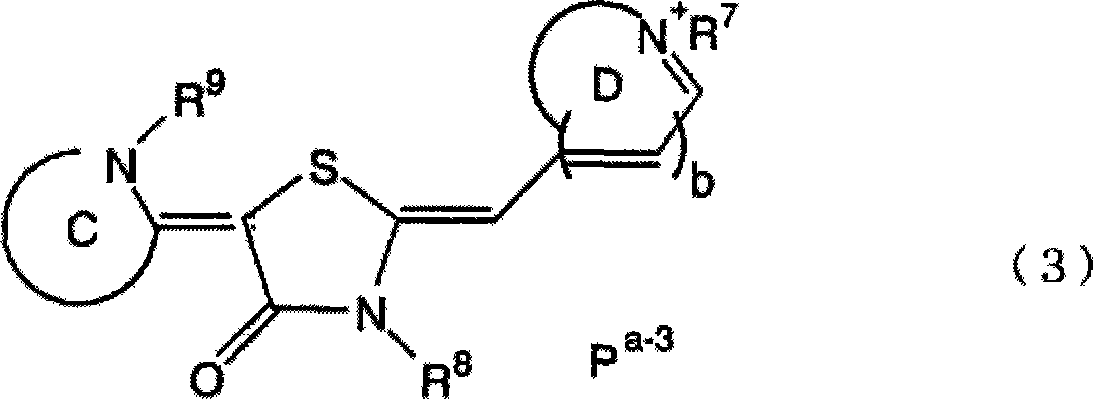
Medicinal composition for prevention or treatment of parasitic protozoan infection
A technology of drugs and compounds, which is applied in the field of pharmaceutical compositions for the prevention or treatment of protozoan infectious diseases, and can solve the problems of not knowing the causal relationship and the difficulty of compound structures, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] 1-1. Cultivation of Drug-Susceptible Tropical Fever Malaria Protozoa
[0064] In this example, a protozoan of Plasmodium falciparum F CR-3 strain was used. The medium used in the experiment was filter-sterilized RPMI-1640 medium, the pH of which was adjusted to 7.4, and human serum was added to a final concentration of 10%. The cultivation of malaria protozoa in O 2 Concentration 5%, CO 2 Concentration 5%, N 2 The concentration is 90%, and the temperature is 36.5°C.
[0065] 1-2. Drug-sensitive Tropical Fever Malaria Protozoan Proliferation Inhibition Screening Test
[0066] The cultured malarial protozoa-infected erythrocytes were collected by centrifugation, washed with serum-containing medium, and then non-infected erythrocytes were added. The initial infection rate was 0.3%. The hematocrit value at this time was 3%. The compound of the present invention used in the test and the positive drug (chloroquine, quinine) were dissolved in dimethyl sulfoxide (DMSO) as...
Embodiment 2
[0087] 2-1. Cultivation of chloroquine-resistant tropical malaria protozoa
[0088] A protozoa of Plasmodium falciparum K1 strain was used in this example. The culture medium used in the experiment is a culture medium formed by adding human serum at a final concentration of 5% to the filter-sterilized RPMI-1640 medium. The cultivation of malaria protozoa in O 2 Concentration 3%, CO 2 Concentration 4%, N 2 The concentration was 93%, and the temperature was 37°C.
[0089] 2-2. Chloroquine-resistant Tropical Fever Malaria Protozoan Proliferation Inhibition Screening Test
[0090] The compound of the present invention used in the test-positive object drug (chloroquine) was dissolved in DMSO to prepare a test solution with a predetermined concentration. The cultured malarial protozoa-infected erythrocytes were collected by centrifugation and diluted with non-infected erythrocytes. The initial infection rate was 0.15%. The hematocrit value at this time was 2.5%. Add 200 μL of...
Embodiment 3
[0111] 3-1. Culture of African trypanosomiasis protozoa
[0112] In this example, the bloodstream-dwelling trypanomastigotes of the protozoa of Trypanosoma brucei rhodensiense (STIB 900 strain) were used. The medium used in the experiment was filter-sterilized MEM medium supplemented with 25mM N-2-hydroxyethylpiperazine-2-ethanesulfonic acid (HEPES), 1g / L glucose, 1% MEM non- Essential amino acids, 0.2mM 2-mercaptoethanol, 2mM sodium pyruvate, 0.1mM hypoxanthine, cold and 15% heat-treated horse serum prepared medium. Protozoa were cultured in CO 2 Concentration of 5% in the air, at a temperature of 37 degrees.
[0113] 3-2. African trypanosomiasis protozoan proliferation inhibition screening test
[0114] The compound of the present invention used in the test and the positive drug (Melarsoprol (Melarsoprol)) were dissolved in dimethyl sulfoxide (DMSO) to prepare a test solution with a predetermined concentration. In the wells of the 96-well culture plate, the number of pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com