Sulfonyl hydrazines as hypoxia-selective antineoplastic agents
A technology selected from and alkoxy groups, applied in the field of sulfonyl hydrazide prodrugs, can solve the problems of insufficient solubility and insolubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-2
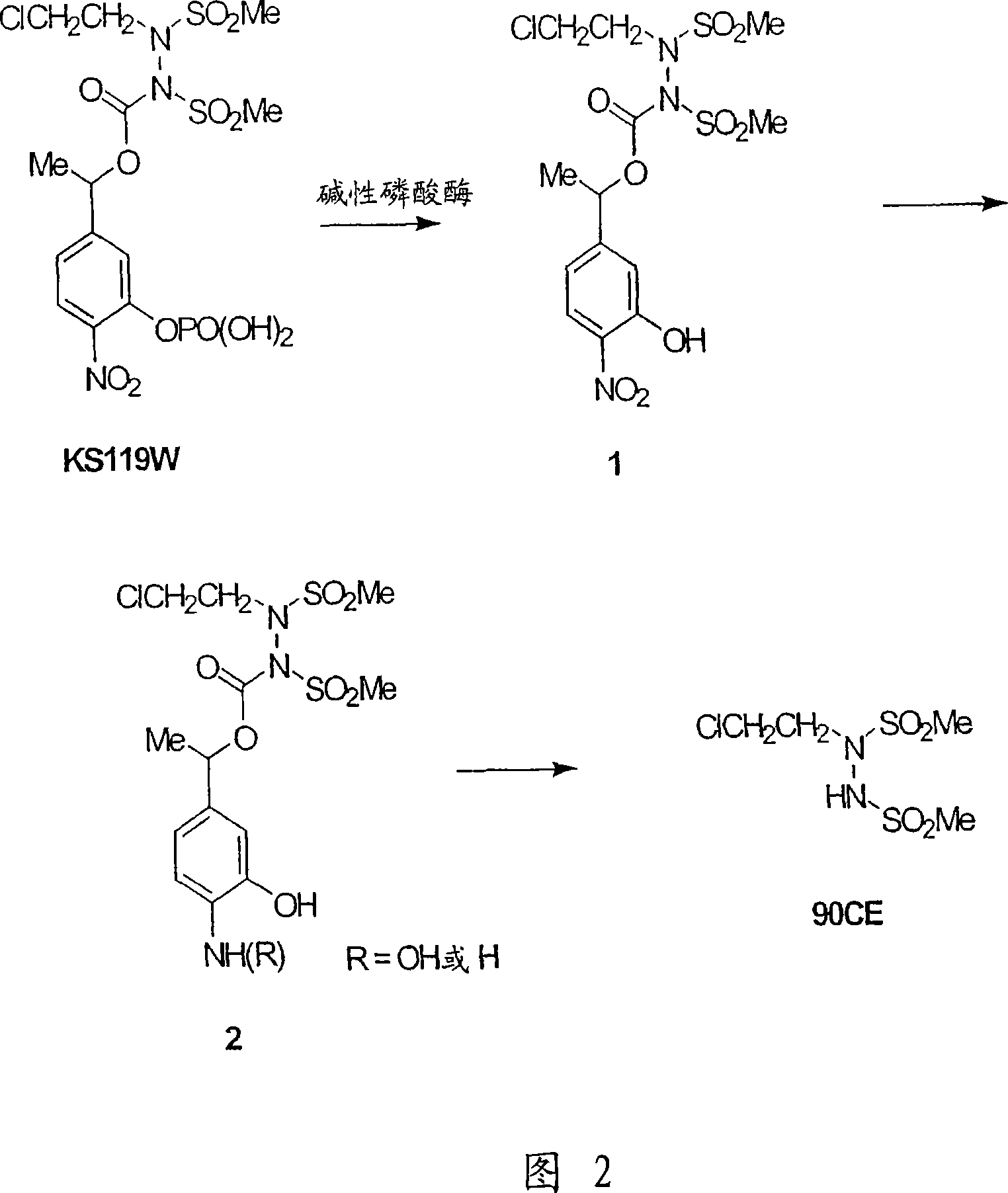
[0109] Phosphorylation of phenolic compounds
[0110] General Method A. To a stirred solution of the appropriate phenolic compound (10.0 mmol) in acetonitrile (15 mL) was added DMAP (1 mmol) and DIPEA (20 mmol) at room temperature. The reaction mixture was cooled to -13°C. A solution of dialkyl chlorophosphate (10 mmol) in acetonitrile (5 mL) was added dropwise to keep the internal temperature below -10°C. The reaction mixture was warmed to 0° C., then stirred continuously for 2 hours, and the completion was monitored by TLC. The reaction mixture was concentrated by rotary evaporation and washed with dichloromethane and 0.5M KHSO 4 aqueous solution to treat the oily residue. The organic layer was passed through anhydrous MgSO 4 Dry, then filter and concentrate to a brown viscous oil. The crude dialkylphosphine carboxy-aryl compound can be used without further purification.
[0111] 1-(3-Diethylphosphinecarboxy-4-nitrophenyl)ethanol (13). Following General Procedure A, ...
Embodiment 3
[0118] Grignard reaction of benzaldehydes
[0119]general method. To a solution of 4-nitrobenzaldehydes (4-nitrobenzaldehydes) (10 mmol) in anhydrous THF (30 mL) at -50 ° C for 45 minutes, slowly add a Grignard reagent, such as diethyl ether of methylmagnesium bromide (25 mmol ) solution. During the addition of the Grignard reagent, the temperature of the reaction mixture was kept below -40°C. The reaction mixture can be warmed to room temperature and stirred for 3.5 hours. The reaction mixture was cooled to -10 °C and quenched with 5% hydrochloric acid (25 mL). The reaction mixture was diluted with water (25 mL) and the product was extracted with ethyl acetate (3 x 15 mL). The combined organic extracts were washed with water to pH 5, passed through anhydrous Na 2 SO 4 Dry, filter and concentrate. The residue was purified by flash chromatography on silica gel, eluting with 25% ethyl acetate in hexanes. After evaporation and drying in vacuo, α-alkyl 4-nitrobenzyl alcoho...
Embodiment 4
[0124] Reduction of acetophenones
[0125] general method. To a solution of chiral (R or S)-2-methyl-CBS-oxazaborlidine (oxazaborlidine) in A (1.0 M in toluene, 240 mL) was added 1.0 M BH 3 -THF solution (120 mL), and the resulting solution was cooled to -50°C. After 4 hours, slowly add 3'-hydroxy-4'-nitroacetophenone (100g) in THF / toluene (200mL / 800mL) solution and 1.0M BH to the above solution 3 - THF solution (1.0 L) while stirring vigorously. The reaction mixture was continuously stirred at -50°C for 2-3 hours, and the completion was monitored by HPLC. Acetone (200 mL) was then added dropwise to the reactor at -50°C. After stirring at -50 °C for 10 min, the reaction mixture was warmed to ambient temperature and stirred for 1.5 h. Concentrate by rotary evaporation on a 45°C bath, and the residue is saturated with Na 2 CO 3 Aqueous solution (2 L) was worked up. The mixture was heated at 50°C for 30 minutes and then cooled to room temperature. Tert-butyl methyl ether...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com