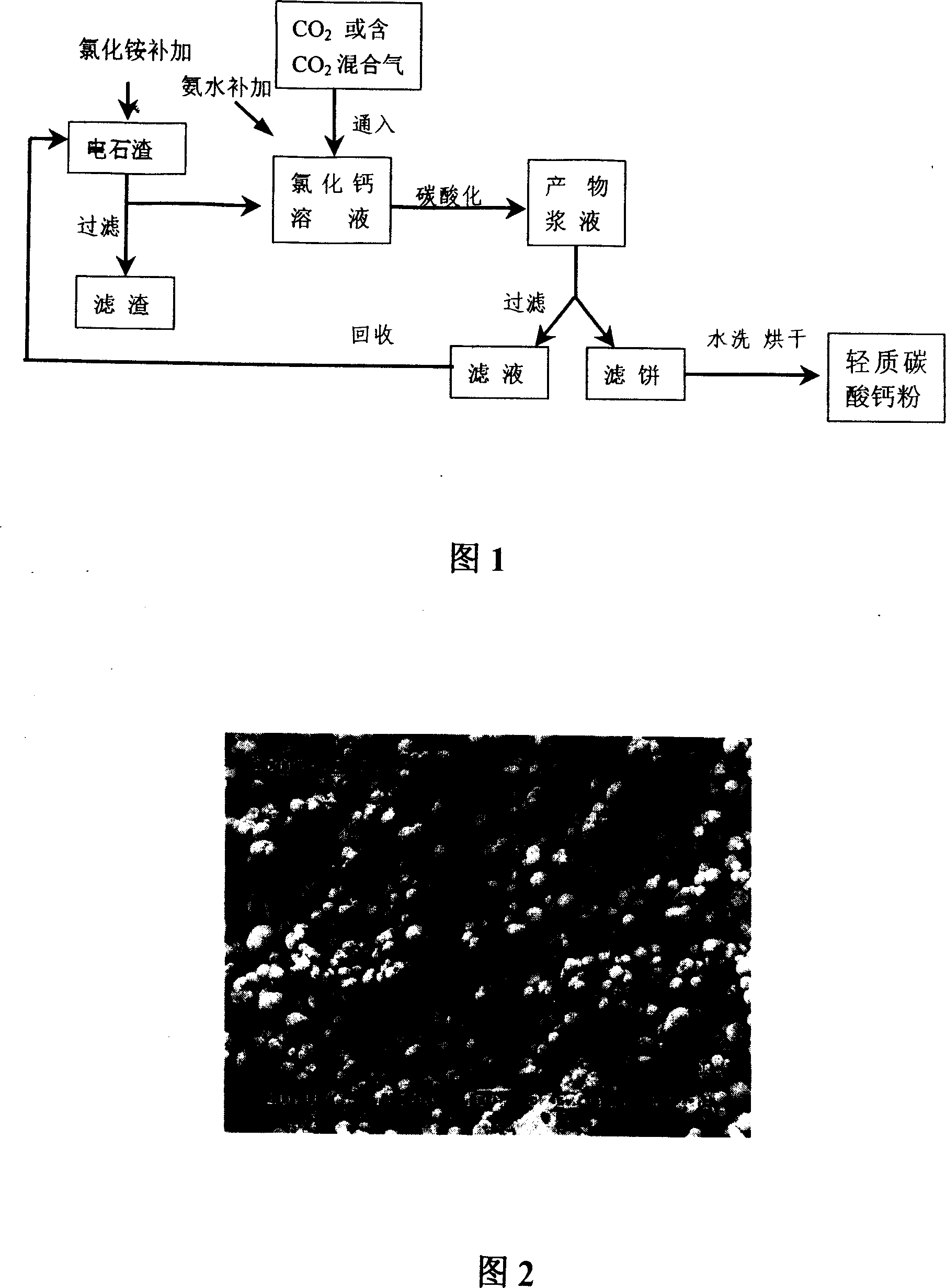
Process of preparing high purity light calcium carbonate fine powder with carbide residue
A technology of calcium carbonate micropowder and carbide slag, applied in the direction of calcium carbonate/strontium/barium, etc., to reduce pollution, reduce loss, and reduce manufacturing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Take 40g of calcium carbide slag (dry basis), add it to ammonium chloride solution with a mass fraction of 20% and an excess of 30%, stir and react at room temperature for 4 hours, filter (or suction filter) with ordinary filter paper, and obtain a clear and transparent Basic CaCl 2 solution. The resulting CaCl 2 The solution was placed in a 1L three-necked flask and diluted with water to a solution concentration of 4%, and stirred at room temperature (stirring speed was 500rpm), while the air flow rate was 800mL / min, and the CO concentration was 600mL / min. 2 Gas flow rate into air and CO 2 The mixed gas starts carbonation reaction, the solution becomes turbid after about 5 minutes, the solution temperature rises gradually, and the system temperature rises to about 30°C after 25 minutes of reaction. The pH value of the solution was measured. The pH value was 10.6 at the initial stage of the reaction, slowly decreased to 8.6 during the reaction, and rapidly decreased ...
Embodiment 2
[0046] Prepare CaCl by carbide slag with embodiment 1 2 After the solution was placed in a 1L three-necked flask, diluted with water to a solution concentration of 10%, stirred at room temperature (stirring speed was 700rpm), while the air flow rate of 1500mL / min, 1000mL / minCO 2 The carbonation reaction was started by feeding the mixed gas at the gas speed, and the pH value of the solution dropped to 7.0 after 21 minutes of reaction, and the reaction was stopped. The product was filtered with suction, washed with water for 1-2 times and dried at 60°C. Observed by a scanning electron microscope, it is uniform spherical particles with a diameter of 2-4 μm, a sedimentation volume of 2.6ml / g, and a whiteness of 97%. Its composition was analyzed by ICP-AES method, and the results are shown in Table 1. It can be seen that the content of heavy metal elements in the product is extremely low, reaching the national food grade calcium carbonate national standard.
Embodiment 3
[0048] After recovering the filtrate after the product filtration in Example 1, measure the nitrogen content of the solution, add a small amount of water and ammonium chloride to make the solution reach the same volume and ammonium chloride concentration as in Example 1. After adding 40g calcium carbide slag in this solution, carry out the processing of carbide slag by the same condition of embodiment 1, the CaCl obtained 2 After solution adds a small amount of ammoniacal liquor, carry out carbonation reaction by the same condition as embodiment 1, product light calcium carbonate micropowder is spherical particle (particle diameter is 1~4 μ m), and the sedimentation volume of product is 2.6ml / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Sedimentation volume | aaaaa | aaaaa |
| Sedimentation volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com