Solid electrolyte material system for all solid state lithium battery and preparation method
A solid electrolyte and all-solid-state technology, applied in batteries with solid electrolytes, non-aqueous electrolyte batteries, secondary battery components, etc., can solve problems such as carrier migration and constraint
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Choose Li 2 S, SiS 2 , AlI3 and LiI powder (or an appropriate molar ratio of Li 2 S, silicon powder, S powder, aluminum powder and iodine particles and LiI powder) are mixed by selected ratio (y=6, m=0.5, z=3, n=1) and carry out batching, then pack into quartz glass tube, Packaged after vacuuming (10 -2 Pa, hydrogen-oxygen fusion seal), carry out solid phase reaction at 500°C-750°C, and the reaction time is 10-14 hours. Take it out at high temperature and quench to room temperature. After the tube is opened, the powder is ground and pressed into tablets (diameter 10mm, thickness about 1mm, pressure 10MPa), and finally made into a lithium ion fast ion conductor test material.
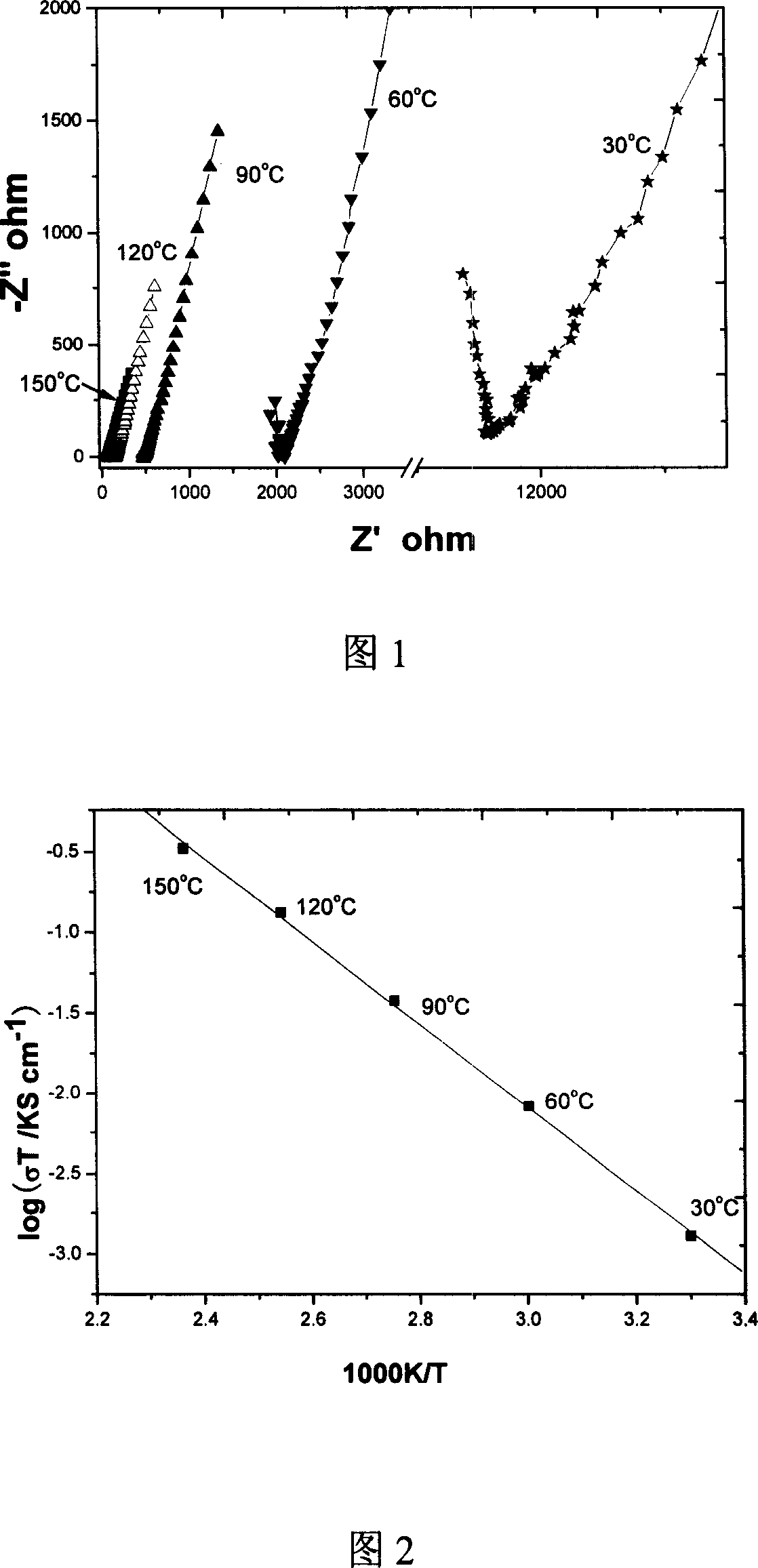
[0024] The conductivity test (see Figure 1) shows that it has ionic conductivity, and the characteristic straight line of ionic conductivity can be clearly observed at the low frequency end of the AC impedance spectrum at different temperatures. By calculation, the total conductivity at room ...
Embodiment 2
[0026] Choose Li 2 S, SiS 2 , AlI 3 The powder is mixed according to the selected ratio (y=6, m=0.5, z=3) for batching, then put into a quartz glass tube, vacuumized and packaged, and the solid phase reaction is carried out at 500 ° C ~ 750 ° C, and the reaction time is 10-14 hours. Take it out at high temperature and quench to room temperature. After the tube is opened, the powder is ground and pressed into tablets (diameter 10mm, thickness about 1mm, pressure 10MPa), and finally made into a lithium ion fast ion conductor test material.
Embodiment 3
[0028] Choose Li 2 S, AlI 3 The powder is mixed according to the selected ratio (x=1.5) for batching, then put into a quartz glass tube, vacuumized and packaged, and solid-phase reaction is carried out at 500°C-750°C, and the reaction time is 10-14 hours. Take it out at high temperature and quench to room temperature. After the tube is opened, the powder is ground and pressed into tablets (diameter 10mm, thickness about 1mm, pressure 10MPa), and finally made into a lithium ion fast ion conductor test material.
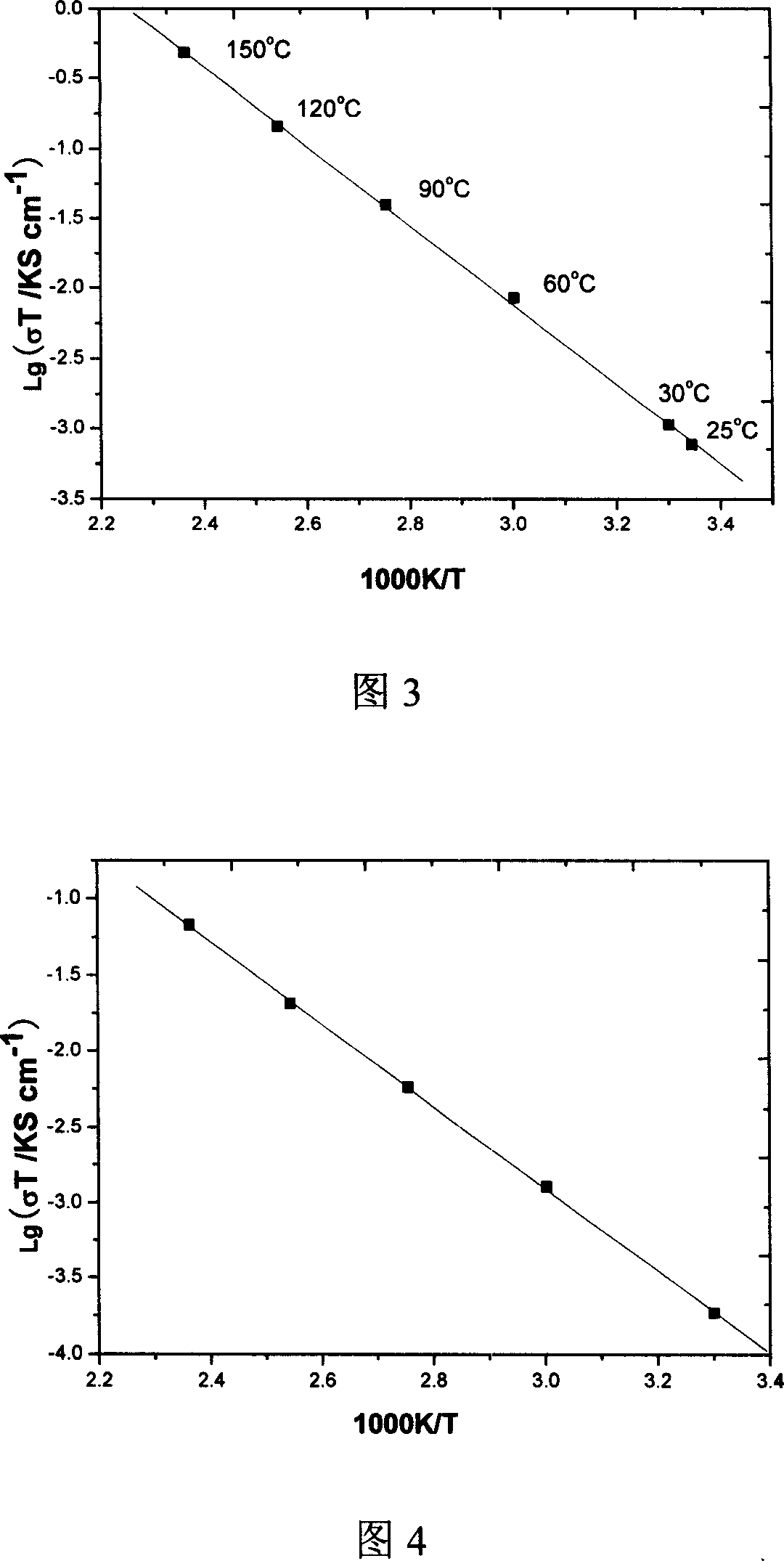
[0029] The conductivity test of the system is shown in Figure 4 as a plot of 1gσT against 1000 / T, and the excellent linearization indicates the ionic conductivity of the system; the calculated ion transport activation energy of the material is (0.53eV). The total conductivity at room temperature is 3.60×10 -7 S / cm, up to 2.36×10 at 150°C -3 S / cm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com