Chip enzymolysis micro-reactor based on functionalized magnetic microsphere and its preparation method and uses
A technology of magnetic microspheres and microreactors, applied in the field of biochemical analysis, can solve the problems of severe synthesis reaction conditions, easy loss of enzyme activity, scrapped enzyme reactors, etc., and achieve high sensitivity, no need for protein denaturants, and easy regeneration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
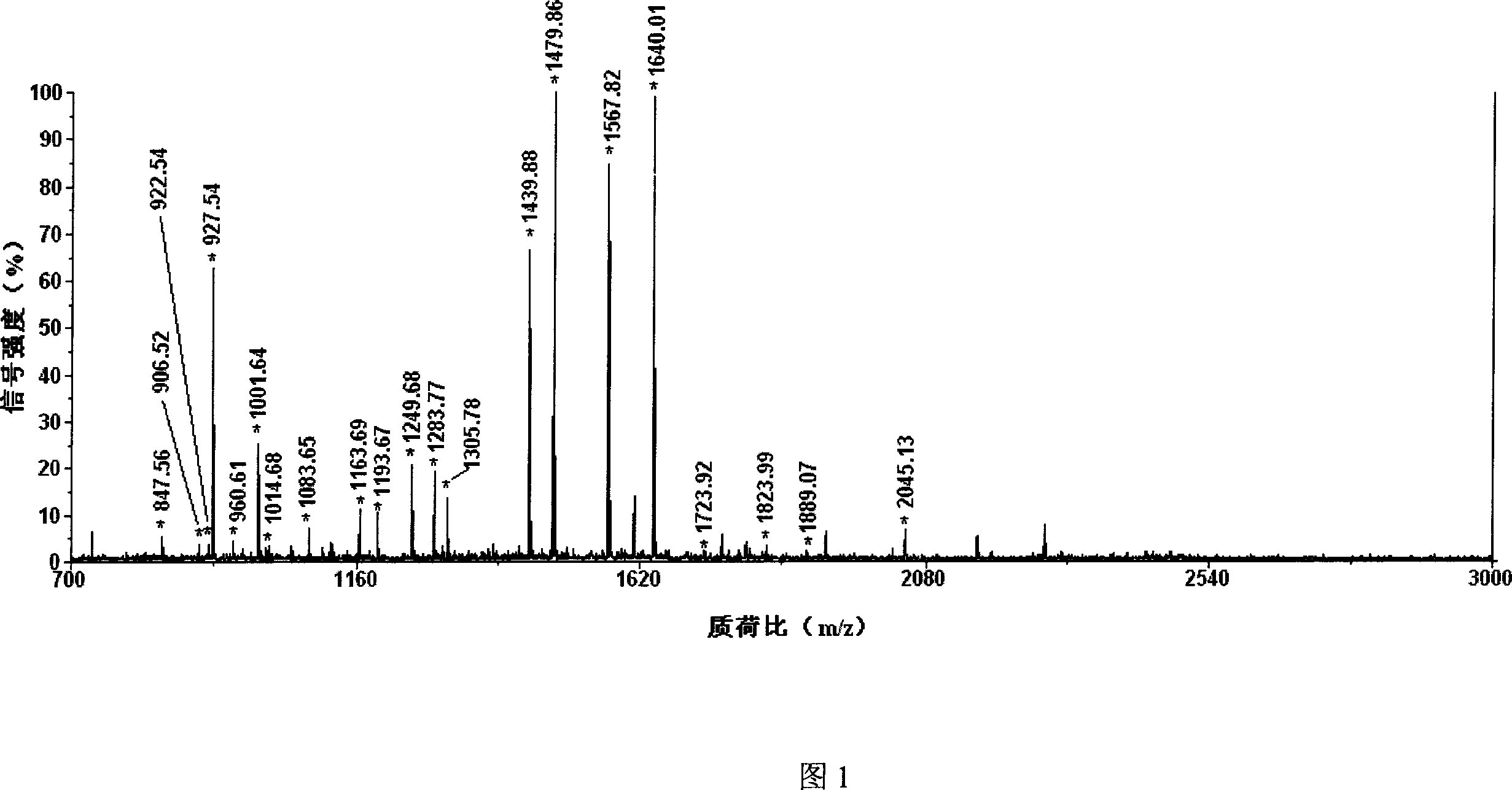
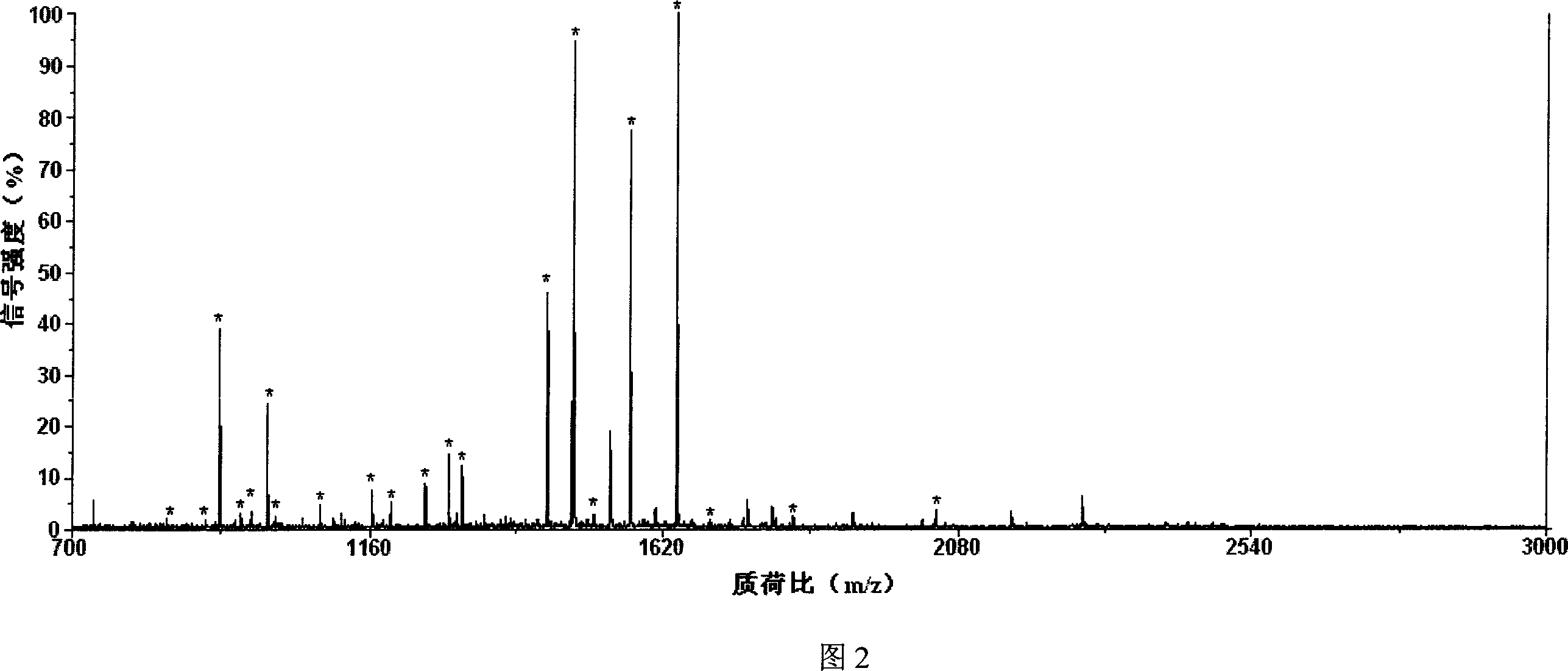
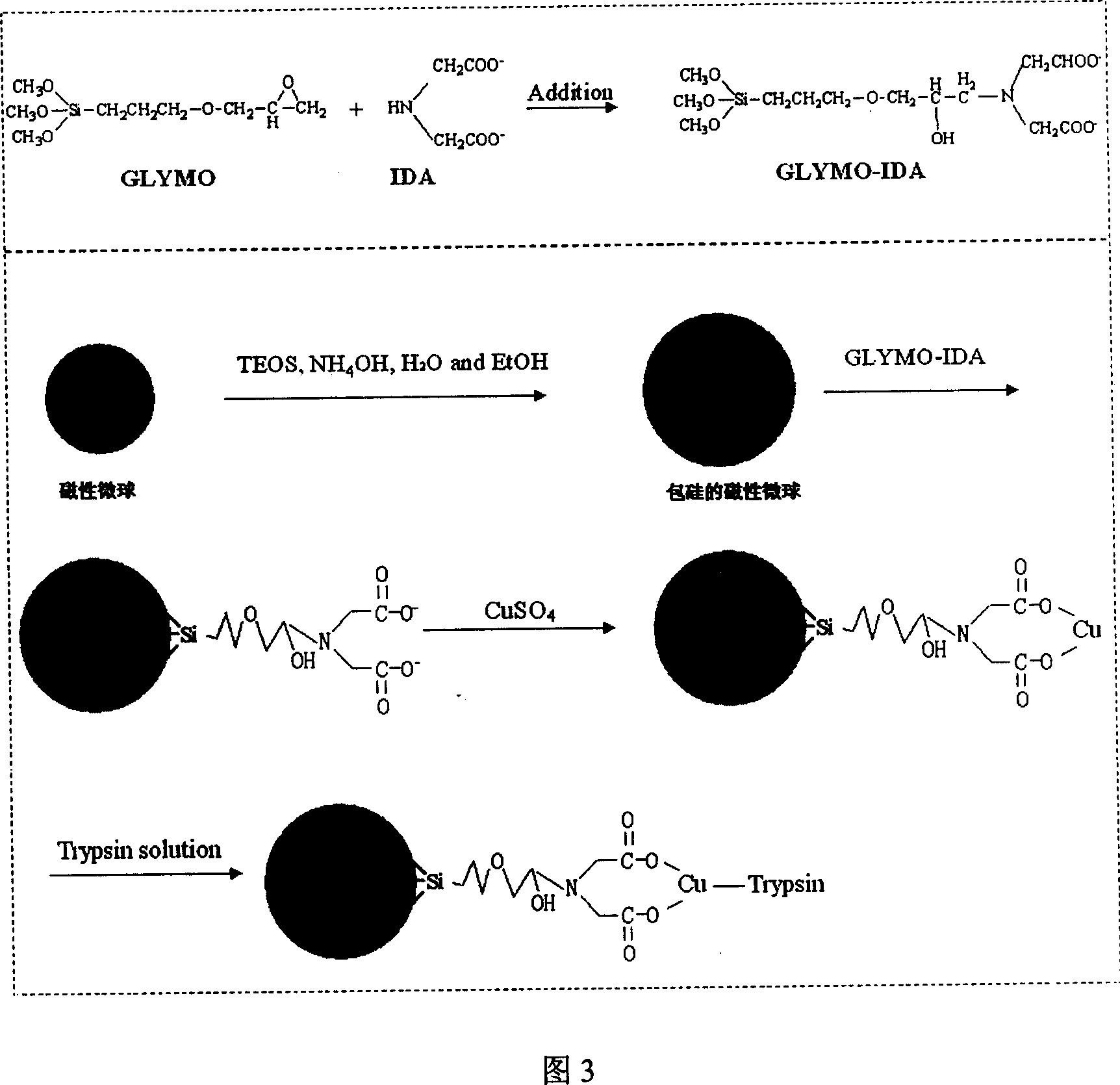
Image
Examples
Embodiment 1
[0040] 1. Preparation of functionalized magnetic microspheres and immobilization of enzymes:
[0041] (1) 2.70 g FeCl 3 ·6H 2 O was dissolved in 100 mL of ethylene glycol under magnetic stirring, and after stirring for 0.5 h, 7.20 g of sodium acetate was added and stirred for another 0.5 h. The obtained solution was transferred to a 200 ml reaction kettle, reacted at 200° C. for 8 hours, and then cooled to room temperature. The ferroferric oxide magnetic microspheres produced by the reaction were collected with a magnet, washed with ethanol and water several times, and the product was vacuum-dried at 60° C. for 12 hours.
[0042] (2) 0.01 gram of ferroferric oxide magnetic microspheres are cleaned with 5 milliliters of 2mol / L hydrochloric acid solution for 5 minutes under ultrasonic, dispersed with 100 mL of ethanol / water (weight ratio 7: 3) solution containing 1% ammonia, and mechanically Under stirring, 0.05 g of ethyl orthosilicate was slowly added into the dispersion of...
Embodiment 2
[0052] 1. Preparation of functionalized magnetic microspheres and immobilization of enzymes:
[0053] (1) 3.8 g FeCl 3 ·6H 2 O was dissolved in 100 mL of ethylene glycol under magnetic stirring, and after stirring for 1 hour, 8.0 g of sodium acetate was added and stirred for another 1 hour. The reaction was carried out at 200° C. for 10 hours. All the other are with embodiment 1.
[0054] (2) 0.02 gram of ferroferric oxide magnetic microspheres were cleaned with 15 milliliters of 2mol / L hydrochloric acid solution for 10 min under ultrasonic, and under mechanical stirring, 0.10 gram of tetraethyl orthosilicate was slowly added in the magnetic microsphere dispersion liquid and heated at room temperature Stirring was continued for 12 hours. The particle size of the magnetic microspheres is 300nm. All the other are with embodiment 1.
[0055] (3) Using tricarboxymethylethylenediamine as a chelating agent, the dosage is 3.00 grams, γ-glycidyl ether propyl trimethoxysilane 1mL...
Embodiment 3
[0062] 1. Preparation of functionalized magnetic microspheres and immobilization of enzymes:
[0063] (1) 2.0 g FeCl 3 ·6H 2 0 was dissolved in 100 ml of ethylene glycol under magnetic stirring, and after stirring for 1 hour, 7.0 g of sodium acetate was added and reacted at 180°C for 6 hours. All the other are with embodiment 1.
[0064] (2) 0.03 g of ferroferric oxide magnetic microspheres were washed with 15 ml of 2 mol / L hydrochloric acid solution for 10 min under ultrasonic, and 0.15 g of ethyl orthosilicate was added under mechanical stirring. The particle size of the magnetic microspheres is 400nm. All the other are with embodiment 2.
[0065] (3) Use nitraminotriacetic acid as a chelating agent, the dosage is 5.00 grams, γ-glycidyl ether propyl trimethoxysilane 1.5mL, the stirring time in the ice-water bath is 12 hours, the heating and stirring time is 8 hours, and the rest are the same Example 1.
[0066] (4) The amount of magnetic microspheres is 0.04 g, the amo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com