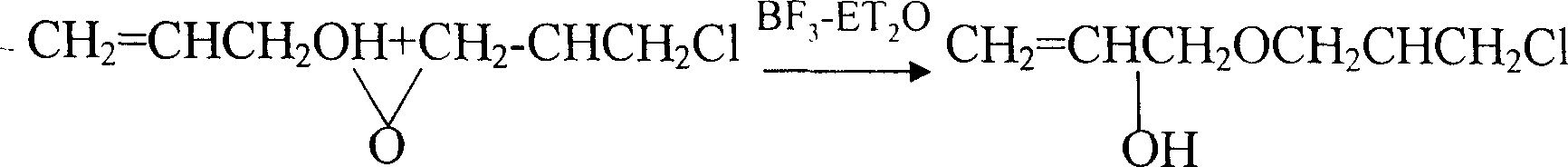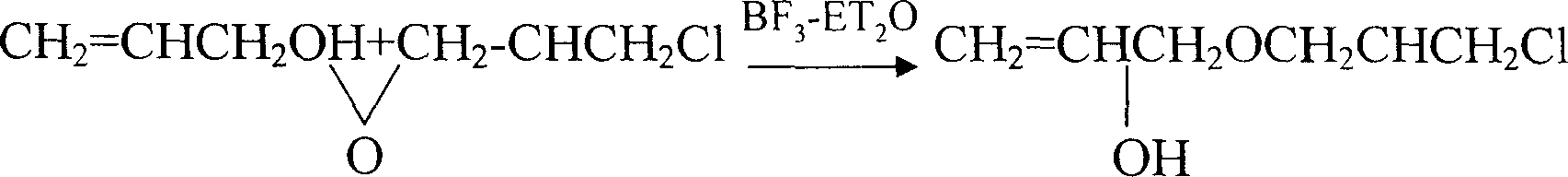Process of industrialized preparing allyl glycidol ether
An allyl glycidyl ether and a technology for preparation steps are applied in the field of preparation of allyl glycidyl ether, and can solve problems such as unfavorable industrialized production, low reaction yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
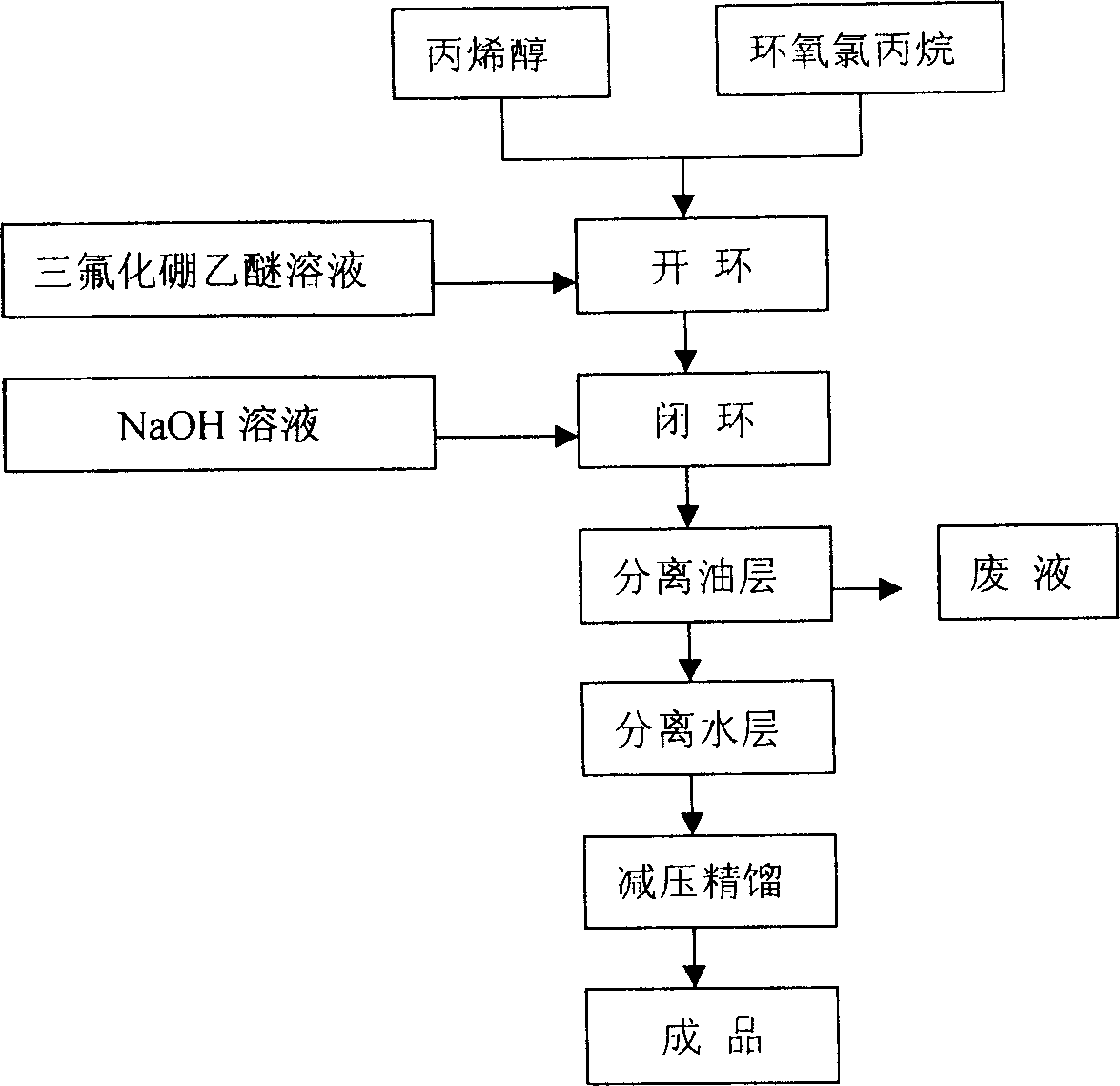
[0015] Concrete preparation steps are as follows:
[0016] The first step of ring opening: add 85kg (1mol) of propenyl alcohol and 600ml (0.007mol) of boron trifluoride ether solution into the batching kettle, stir well and preheat the reaction solution to 50℃~55℃, then add epoxy chlorine Propane 120kg (1.4mol) controls the flow rate, gradually heats up the temperature, collects the front fraction when the temperature of the material in the kettle rises to 130°C, and sets the temperature of the temperature controller at 160°C, and turns off the power when the temperature of the material rises to 142°C At this time, the reaction temperature will continue to rise to about 146°C, indicating that the condensation ring-opening reaction is successful. When the temperature of the material in the kettle drops to 120°C, 190kg of distillate is collected, and the refractive index of the distillate is: n 25 D 1.4625±0.0005.
[0017] Reaction structure:
[0018]
[0019] The second s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com