Application of turmeric element in the preparation of medicament for restraining uric acid transportor URAT1
A technology of curcumin and transporter is applied in the application field of curcumin in the preparation of uric acid transporter URAT1 inhibitory drugs, and achieves the effects of clear action target, good safety and definite active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
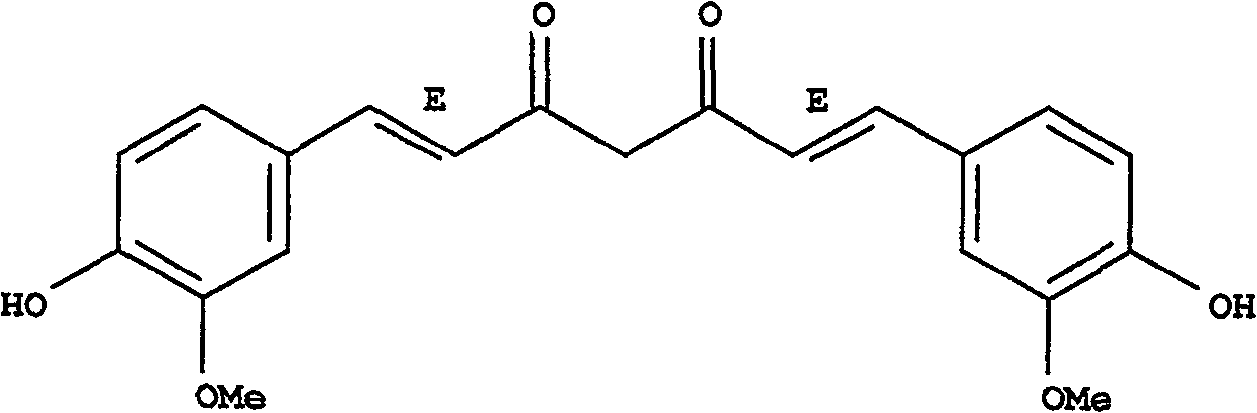
[0015] Example 1: Inhibition and regulation of flavonoid monomer compound curcumin on uric acid transporter URAT1
[0016] Experimental animals: Kunming mice, 15-20 grams, male
[0017] Drug preparation: curcumin is uniformly dispersed in normal saline for intragastric administration, and the dosage is 25mg / kg
[0018] Experimental materials: Trizol Reagment (Invigen), chloroform, isopropanol, absolute ethanol, DEPC, M-MLV
[0019] Reverse transcriptase, PCR kit, RIPA lysate, etc.
[0020] Experimental equipment: high-speed refrigerated centrifuge, high-speed homogenizer, Bio-rad vertical electrophoresis tank, MBI-PCR instrument, horizontal electrophoresis tank, etc.
[0021] Experimental model: mouse hyperuricemia model.
[0022] experimental method:
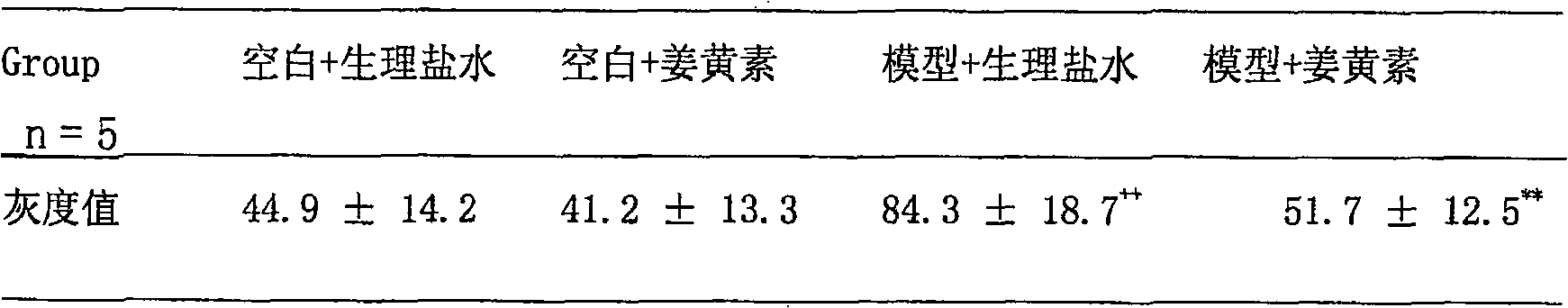
[0023] 1. Establishment of the model: After one week of adaptation to the environment, the animals were given oxonic acid potassium salt 250 mg / kg by intragastric administration; the control group was given an equal volume ...
Embodiment 2
[0038] The monomer compound curcumin is dissolved in water and an appropriate amount of solubilizer (polyethylene glycol 400) according to the conventional preparation method, subpackaged, sterilized, and prepared into a curcumin oral liquid whose specification is 20 mg / ml;
[0039] The monomer compound curcumin is prepared into curcumin capsules with a specification of 20 mg / grain according to the conventional preparation method, and the soft capsule material is selected from gelatin and sorbitol;
[0040] The monomer compound curcumin was prepared according to the conventional preparation method, and the excipient cyclodextrin was added, mixed uniformly, granulated, compressed into tablets, and prepared into curcumin tablets with a specification of 20 mg / tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com