Method for preparing lithium hexafluorophosphate
A technology of lithium hexafluorophosphate and lithium fluoride, which is applied in chemical instruments and methods, lithium compounds, phosphorus compounds, etc., can solve the problems of difficult raw materials and high production costs, and achieve the effect of low price and easy raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
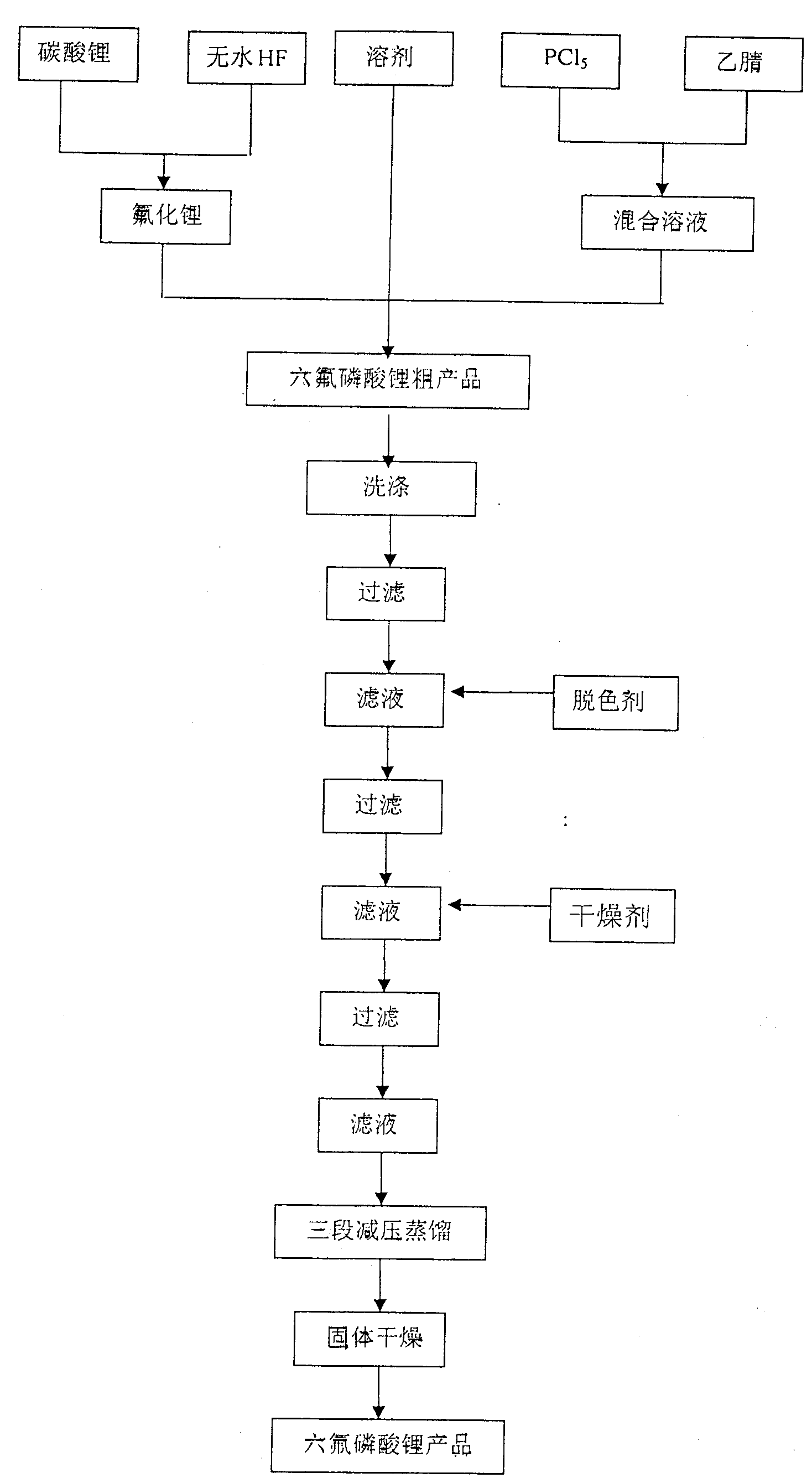
[0014] The preparation method of lithium hexafluorophosphate provided by the present invention comprises reacting lithium fluoride with a phosphorus source, wherein the lithium fluoride is prepared by reacting lithium carbonate and hydrogen fluoride.
[0015] The lithium carbonate can be obtained commercially, preferably industrial grade or battery grade lithium carbonate. It is also possible to separate and purify commercially pure lithium carbonate by various methods such as washing and recrystallization to obtain high-purity lithium carbonate with a purity greater than 99.9%.
[0016] If the reaction system for preparing lithium hexafluorophosphate contains water, lithium oxyphosphate (LiPO x f y ) by-product, lithium oxyphosphate will be partially decomposed into LiF, and LiF will become an impurity in lithium hexafluorophosphate. Therefore, the moisture content in the reaction system for preparing lithium hexafluorophosphate should be as low as possible. In addition, i...
Embodiment 1
[0029] This example is used to illustrate the preparation method of lithium hexafluorophosphate provided by the present invention.
[0030] 111 g Li 2 CO 3 , 40 grams of anhydrous HF are added to 1000 milliliters of PTFE-coated bell-type vacuum equipment (produced by Tianyuan Vacuum Equipment Technology Co., Ltd., Zhongshan City, Guangdong Province), and N 2 The air in the container was replaced, the temperature of the reaction system was controlled at 100° C., the vacuum degree was controlled at 0.07 MPa, and stirring was carried out for 8 hours under hot air circulation. Then, 1000 milliliters of DMC was passed into the container by atomization method, and after continuing to stir for 2 hours, the system was cooled to 45° C. with cooling water, and then the mixture containing 102 grams of PCl 5 150 ml of acetonitrile solution was slowly added dropwise to the reaction vessel, and the drop was completed in 40 minutes, and the stirring was continued at 45° C. for 2 hours.
...
Embodiment 2
[0033] 74 g Li 2 CO 3 , 160.0 grams of anhydrous HF was added to a 1000 ml airtight container coated with PTFE, and N 2 The air in the container was replaced, the temperature of the reaction system was controlled at 125° C., and the mixture was stirred for 3.5 hours under the circulation of hot air flow. Then, 400 milliliters of DMC was passed into the container by atomization method, and after continuing to stir for 2 hours, the system was cooled to 70° C. with cooling water, and then the mixture containing 208 grams of PCl 5 1000 ml of acetonitrile solution was slowly added dropwise to the reaction vessel, and the drop was completed in 40 minutes, and the stirring was continued at 40° C. for 2 hours.
[0034] The above system was filtered and the residue was washed three times with 50 ml of DMC each time. After the filtrates were combined, 35 g of activated carbon was added, stirred at room temperature for 2 hours, and filtered. Add 50 g of 5A molecular sieves to the fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com