Ultra-branched polymer used as polythene processing auxiliary agent and synthetic method
A technology of hyperbranched polymers and processing aids, applied in the field of hyperbranched polymers, can solve the problems of low molecular weight, reduced melt viscosity of ultra-high molecular weight polyethylene, single structure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
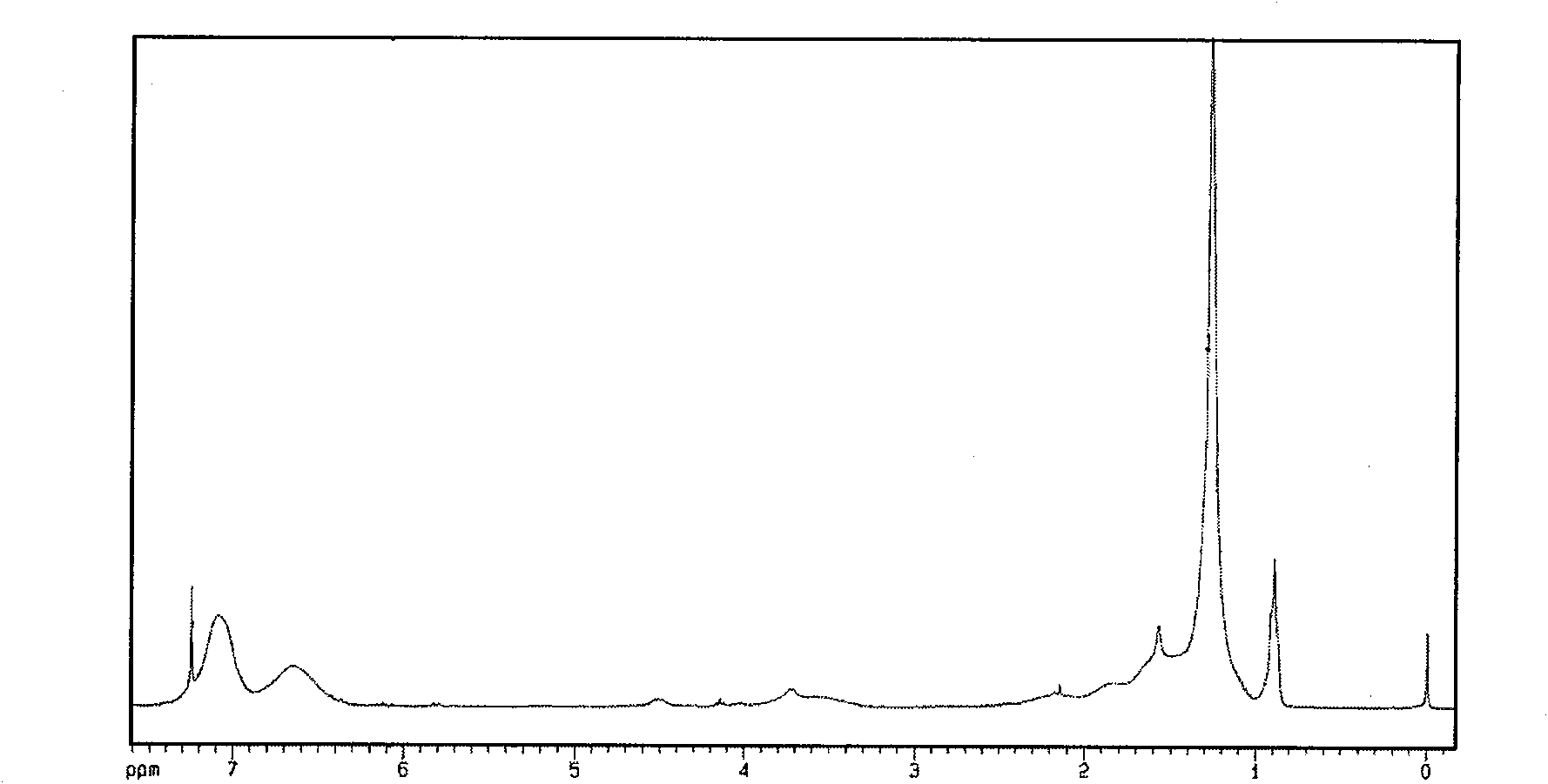
[0019] 5.08g (0.0333mol) of p-chloromethylstyrene, 39.96g (0.167mol) of lauryl acrylate, 34.66g (0.3333mol) of styrene, 0.495g (0.005mol) of cuprous chloride, and 1.56 g of bipyridine g (0.01mol) was added to a 250mL round-bottomed flask, and reacted at 110°C for 8 hours under magnetic stirring. Dry under vacuum for 48 hours to obtain the target hyperbranched polymer (HBP), with a yield of 75%. hyperbranched polymer 1 H-NMR chart see attached figure 1 ,according to 1 The H-NMR chart calculates that the molar content of lauryl acrylate structural unit in the copolymer is 30%, which is close to the monomer composition of 31.3% when feeding. The product was measured by gel permeation chromatography (GPC). w,GPC =9.4×10 4 ,M n,GPC =3.4×10 4 , M measured by multi-angle laser light scattering (MALLS) w,MALLS =1.3×10 5 . The absolute weight-average molecular weight measured by light scattering is significantly greater than the relative weight-average molecular weight measur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com