Stabilized nalmefene hydrochloride injection and its preparation
A technology for injection and nalmefene, applied in the field of nalmefene injection and its preparation, can solve the problems of cumbersome clinical use, increase production cost and the like, and achieve the effects of reasonable formulation, good stability and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] prescription:
[0023] Nalmefene Hydrochloride 0.1g
[0025] Proper amount of hydrochloric acid to pH3.6
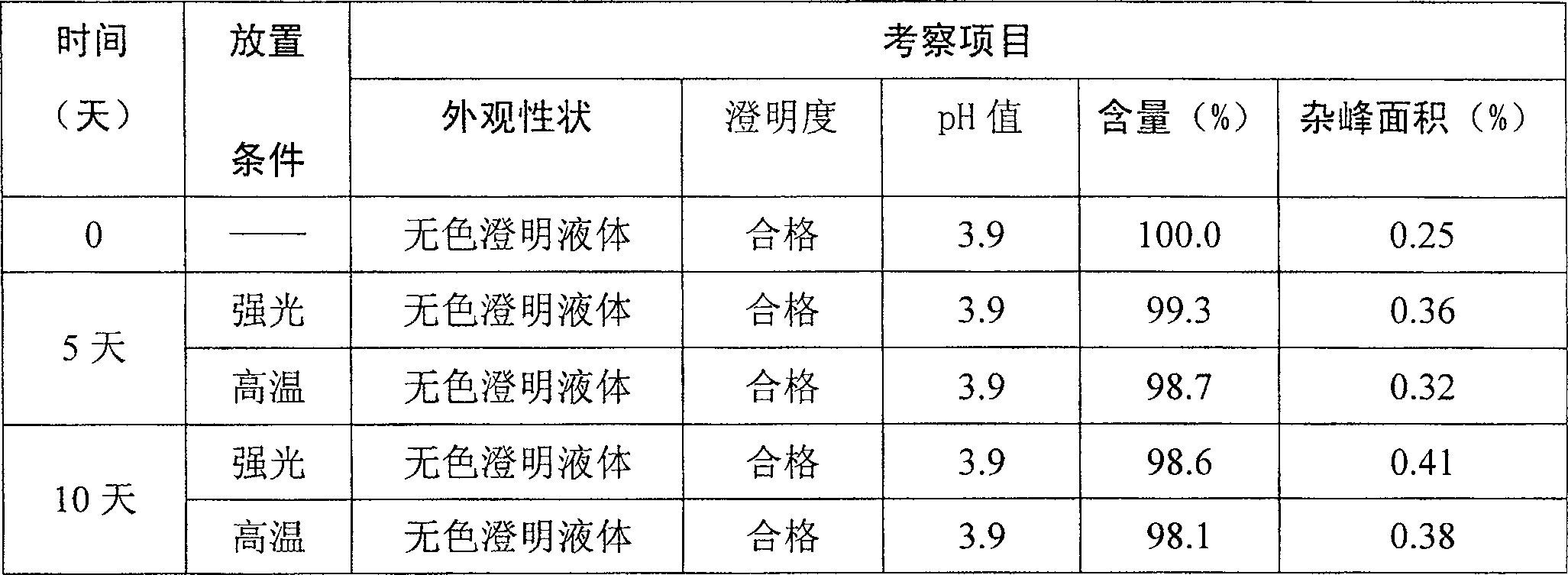
[0026] Process: 1) Take 1000ml of water for injection and put it in the liquid mixing tank, add the full amount of sodium chloride prescribed, and stir until dissolved; 2) Add the prescribed amount of nalmefene hydrochloride, stir until dissolved; then use a 0.22 μm microporous filter membrane Fine filtration until clarity; 3) filling in a glass ampoule (filling 1ml of liquid in a glass ampoule with a volume of 2ml); 4) melting and sealing. 5) Sterilize at 115°C for 20 minutes. 5) Capping, labeling, packaging, and the finished product after passing the inspection. PH value after sterilization: Take 10 sticks of this product, combine them, and measure according to the law (Appendix VI H of Part Two of the Chinese Pharmacopoeia Edition in 2000), and the pH value is 3.9.
Embodiment 2
[0028] prescription:
[0029] Nalmefene Hydrochloride 1g
[0030] Sodium chloride 9g
[0031] Proper amount of hydrochloric acid to pH3.8
[0032] Technology: with embodiment 1. 2ml glass ampoules are used for dispensing, and the dispensing volume is 2ml.
[0033] PH value: measuring method is the same as embodiment 1, gets the solution in 5 ampoules to merge, and the result of measuring pH value is 3.9.
Embodiment 3
[0035] prescription:
[0036] Nalmefene Hydrochloride 1g
[0037] Sodium chloride 9g
[0038] Proper amount of hydrochloric acid to pH3.8
[0039] Technology: with embodiment 1. 5ml glass ampoules are used for dispensing, and the dispensing volume is 4ml.
[0040] PH value: measuring method is the same as embodiment 1, gets the solution in 3 ampoules to merge, and the result of measuring pH value is 3.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com