Anticancer injection of rabdosia diterpene compound or its derivative and its preparing method
A technology for Rubescensica and diterpenoids, which is applied in the field of preparation of Rubesia officinalis diterpenoid compounds or derivatives thereof for injection with anticancer effect, can solve the problems of low bioavailability, insufficient safety, poor stability and the like, To achieve the effect of improving solubility, good stability and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Oridonium diterpenoid injection preparation
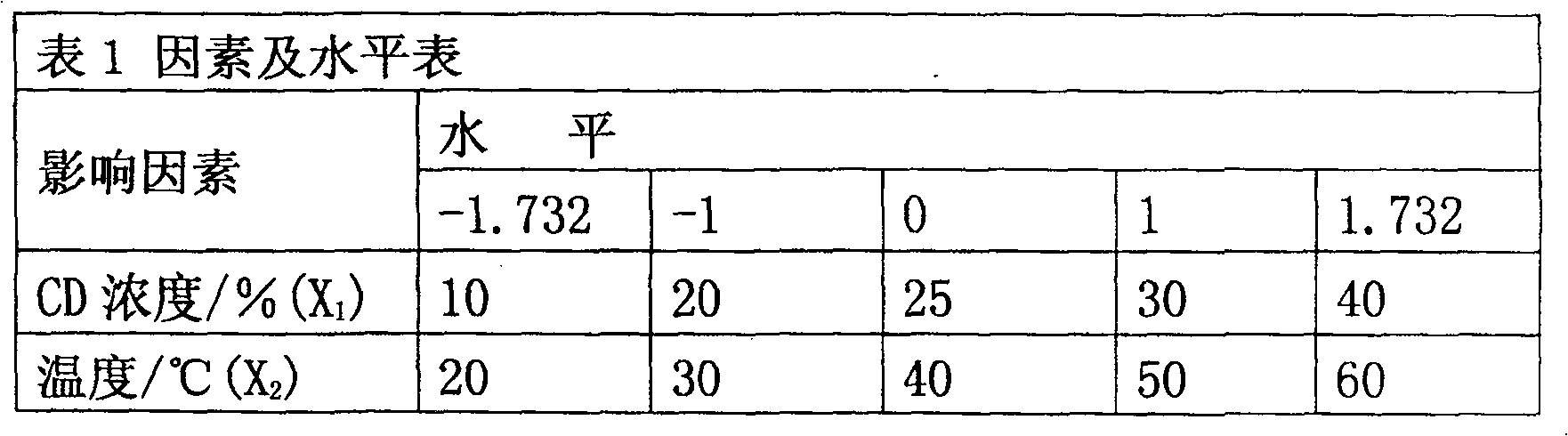
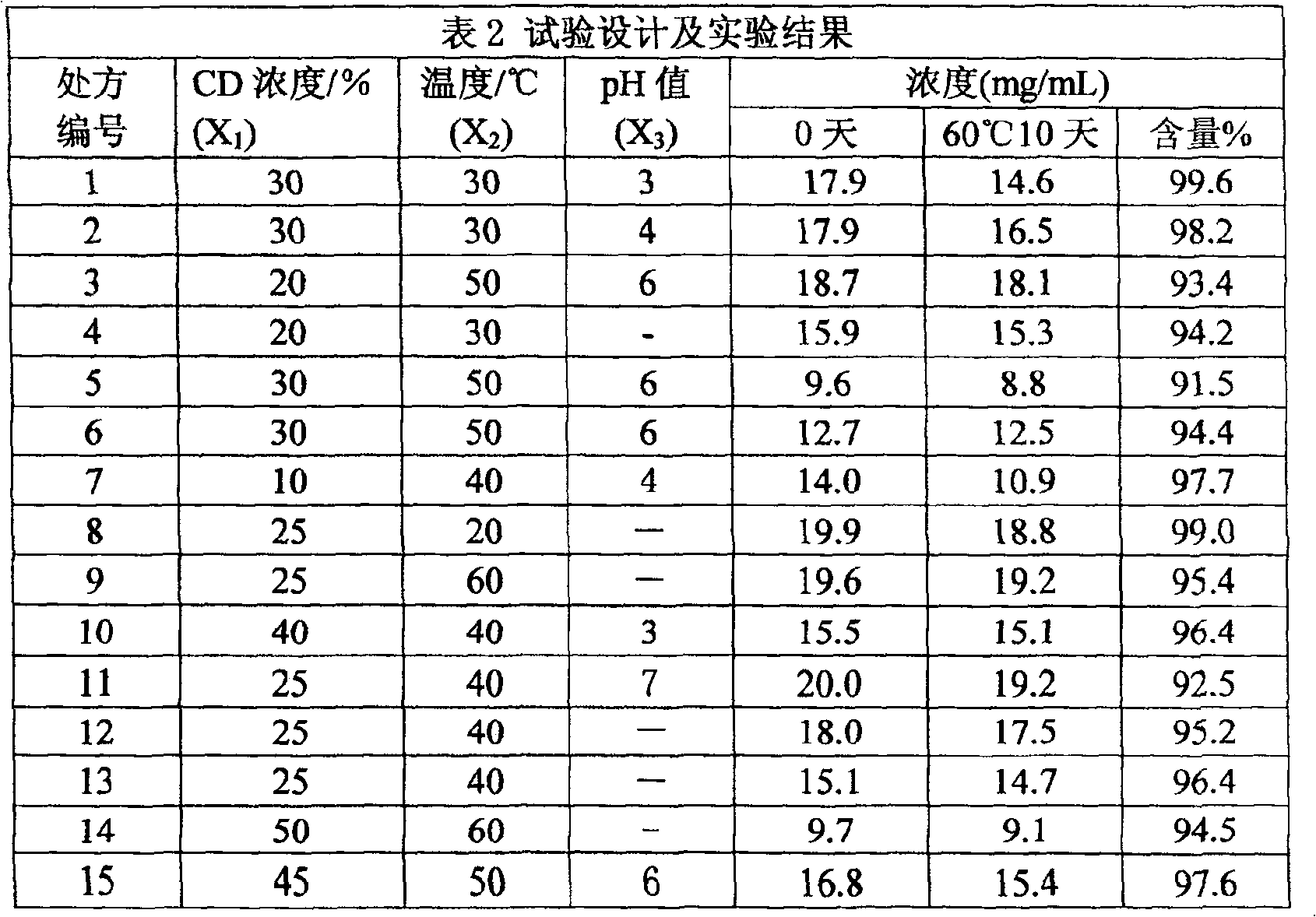
[0031] The amount of raw material components (g): 0.5g of raw materials oridonin, oridonin B, oridonin C, oridonin, oridonin or oridonin, solubilizer β - Cyclodextrin 2-HP-β-CYD 10g, the remaining amount of water for injection to 50ml.
[0032] Preparation process: Weigh the raw material medicine of the prescribed amount, pulverize it and pass through an 80-mesh sieve, and set aside; in addition, fully dissolve the prescribed amount of 2HP-β-CYD with distilled water under continuous stirring, add the raw material drug to the above solution, and use distilled water The volume was adjusted to 50ml, the pH was adjusted to 4, magnetically stirred at 30°C and 50rpm / min for 5 hours, and left at room temperature to balance overnight. Filter the medicinal solution into a sterile container with a microporous filter membrane with a pore size of 0.2um, fill the medicinal solution into a pre-sterilized ampoule, seal and sterilize.
Embodiment 2
[0034] Oridonium diterpenoid injection preparation
[0035] Raw material ingredient dosage (g): 0.25g of raw material drug oridonin, oridonin B, oridonin C, oridonin, oridonin or oridonin, solubilizer hydroxy Propyl-β-cyclodextrin, hydroxyethyl-β-cyclodextrin, trimethyl-β-cyclodextrin or sulfoalkyl ether-β-cyclodextrin 6g, the balance of water for injection to 20ml.
[0036] Preparation process: Weigh the prescribed amount of raw material medicine, pulverize it and pass it through an 80-mesh sieve, and set aside; take another prescribed amount of solubilizer β-cyclodextrin, fully dissolve it with distilled water under continuous stirring, and add the treated raw material medicine The above solution was adjusted to 20ml with distilled water, adjusted to pH 6, magnetically stirred at 40°C and 30rpm / min for 4 hours, left to stand at room temperature for 6 hours, filtered with a 0.2um nylon microporous membrane, and the filtrate was used as a mobile phase After diluting 4 times, ...
Embodiment 3
[0038] Oridonium diterpenoid injection preparation
[0039]Raw material ingredient dosage (g): 0.3g of raw material drug oridonin, oridonin B, oridonin C, oridonin, oridonin or oridonin, solubilizer hydroxy Propyl-β-cyclodextrin, hydroxyethyl-β-cyclodextrin, trimethyl-β-cyclodextrin or sulfoalkyl ether-β-cyclodextrin 5g, the balance of water for injection to 20ml.
[0040] Preparation process: Weigh the prescribed amount of raw material medicine, pulverize it and pass through a 100-mesh sieve, and set aside; in addition, fully dissolve the prescribed amount of SEB-β-CYD with distilled water under continuous stirring, add the raw material drug to the above solution, and use distilled water The volume was adjusted to 20ml, the pH was adjusted to 3, magnetically stirred at 60°C and 50rpm / min for 4 hours, and allowed to stand at room temperature for 6 hours to balance. Filter the medicinal solution into a sterile container with a microporous filter membrane with a pore size of 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com