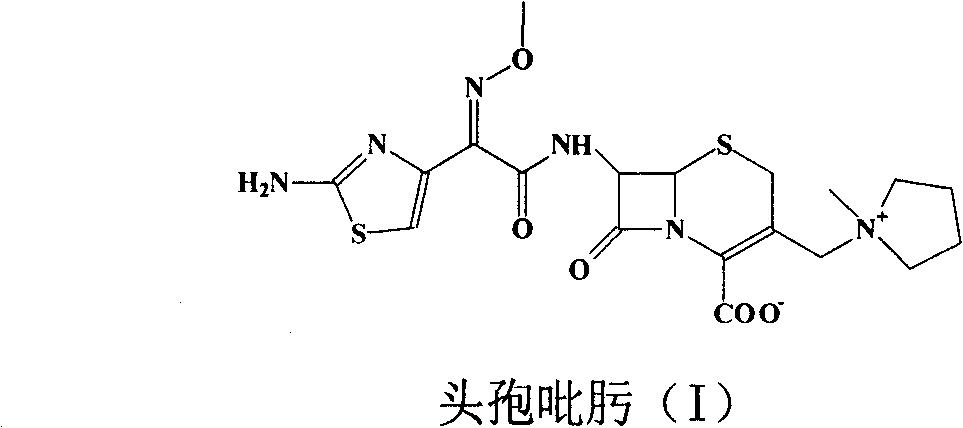
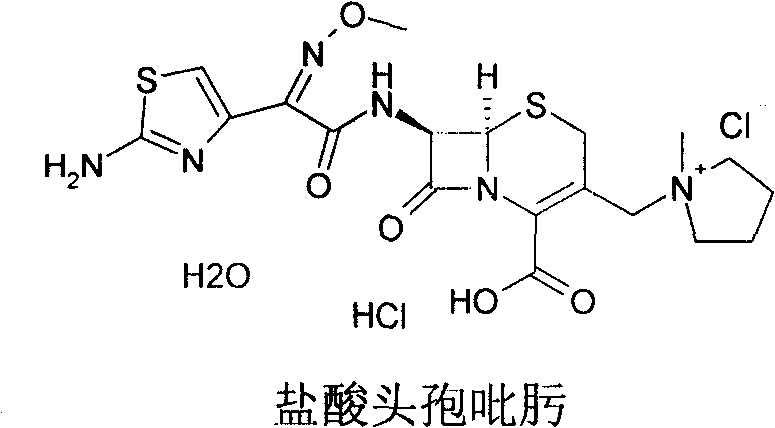
Cefepime halogen acid salt preparation method
A technology of hydrohalide salts and cefepime, which is applied in the field of preparation of cephalosporin compounds, can solve problems such as reducing production costs, and achieve the effects of reducing production costs, improving purity, and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Dissolve 8g of barium chloride dihydrate (32.8mmol, which is 0.95 times the molar ratio of cefepime sulfate) in 70mL of water at room temperature, and then add 20g of cefepime sulfate (34.5mmol, weight by anhydrous ) to form a slurry system with good fluidity. After 10 minutes, add an appropriate amount of activated carbon and stir for 30 minutes. The solid was isolated by centrifugation or suction filtration. Wash the filter cake with an appropriate amount of water, and combine the filtrates. 600 mL of acetone was added to the filtrate under stirring, and the addition was completed within about 1 hour. The temperature of the system was lowered to 0-5°C again, and the stirring was continued for 1 hour. Suction. The filter cake was washed with acetone and dried under vacuum at 40°C to obtain about 18 g of white crystalline powder. The purity is 99.7% (HPLC), the water content is 4.0% (K-F method), the residual acetone is 0.31% (gas chromatography), and the pH of the...
Embodiment 2
[0034]Dissolve 8g of barium chloride dihydrate (32.8mmol) in 60mL of water at room temperature, and then add 20g of cefepime sulfate (34.5mmol, weight based on anhydrous matter) in batches under effective stirring to form a slurry system with good fluidity . After 10 minutes, add an appropriate amount of activated carbon and 30 mL of methanol, and stir for 30 minutes. The solid was isolated by centrifugation or suction filtration. Wash the filter cake with an appropriate amount of methanol-water 1:1 (v / v) mixed solution, and combine the filtrates. 600 mL of acetone was added to the filtrate under stirring, and the addition was completed within about 1 hour. The temperature of the system was lowered to 0-5°C again, and the stirring was continued for 1 hour. Suction. The filter cake was washed with acetone and dried under vacuum at 40°C to obtain about 17.5 g of white crystalline powder. The water content is 3.8% (K-F method), the remaining acetone is 0.35%, and the remaini...
Embodiment 3
[0036] Dissolve 8g of barium chloride dihydrate (32.8mmol) in 60mL of water at room temperature, and then add 30mL of methanol. Under effective stirring, 20 g of cefepime sulfate (34.5 mmol, calculated as anhydrous matter) was added in batches to form a slurry system with good fluidity. After stirring for 30 minutes, the solid was separated by centrifugation or suction filtration. Wash the filter cake with an appropriate amount of methanol-water 1:1 (v / v) mixed solution, and combine the filtrates. 600 mL of acetone was added to the filtrate under stirring, and the addition was completed within about 1 hour. The temperature of the system was lowered to 0-5°C again, and the stirring was continued for 1 hour. Suction. The filter cake was washed with acetone and dried under vacuum at 40°C to obtain about 17.5 g of white crystalline powder. Content and water content data show that the product is also cefepime dihydrochloride monohydrate. Acetone remained 0.30%, methanol remain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com