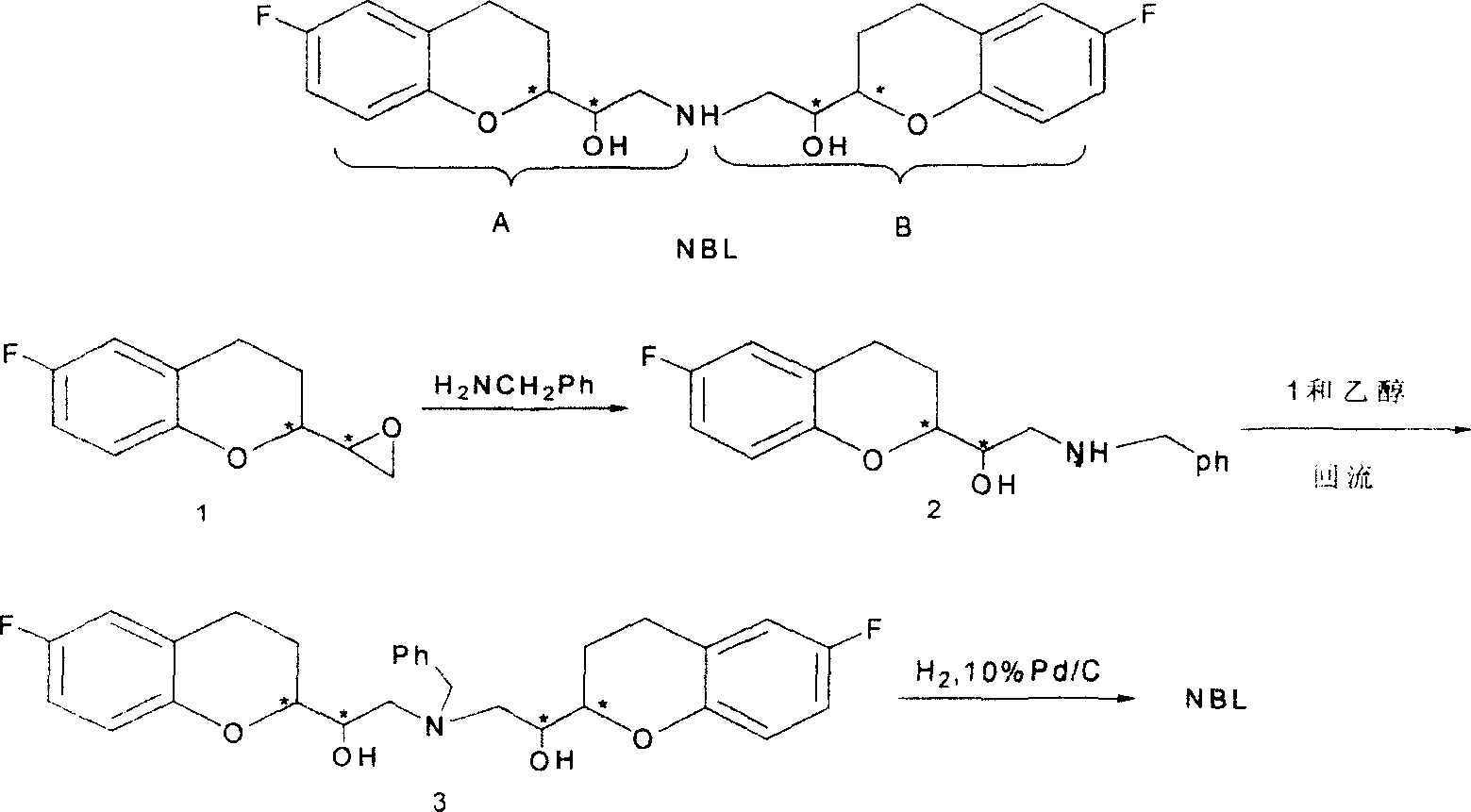
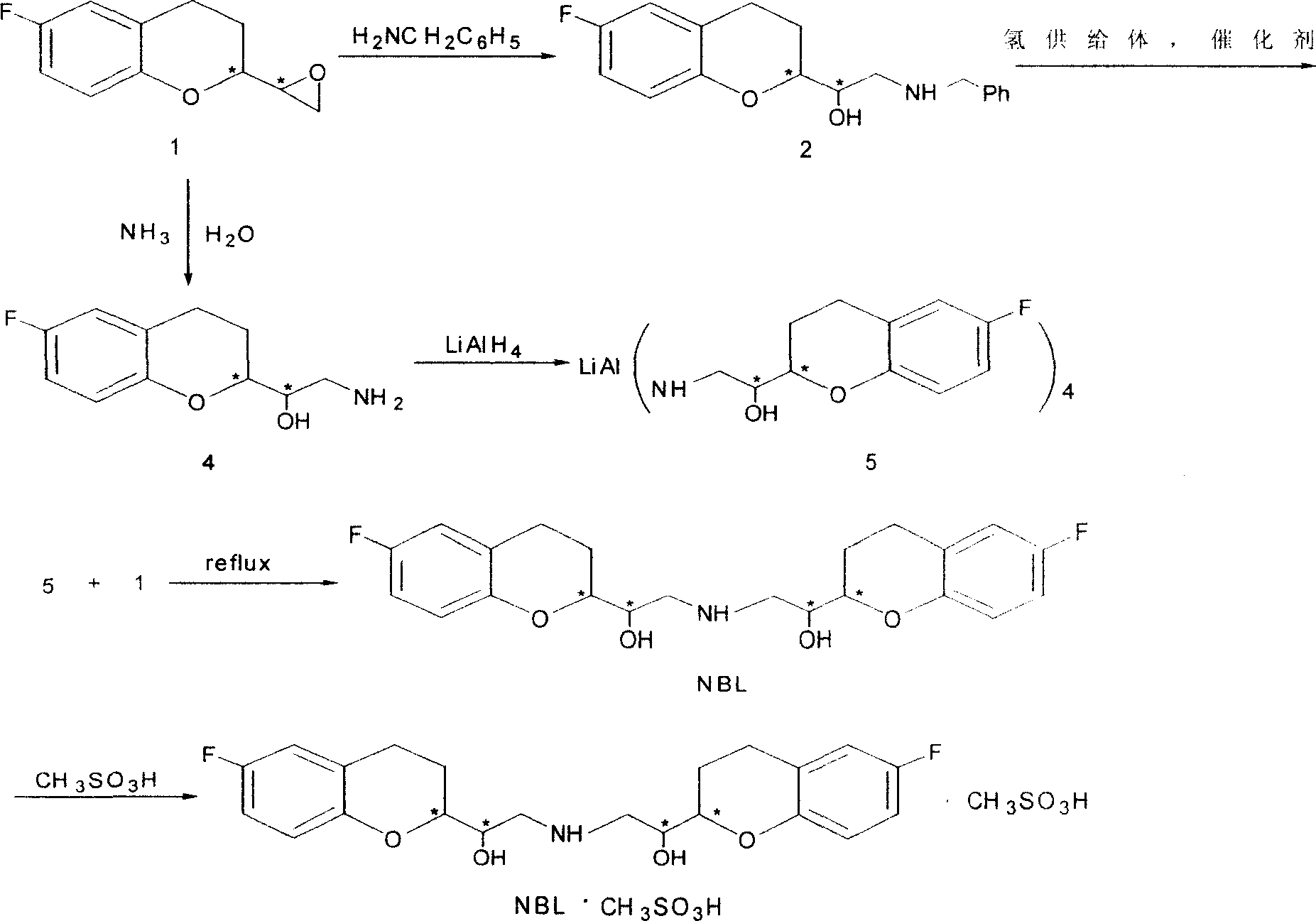
2,2'-[iminodi(methylene)di-(6-fluoro-3,4-dihydro-2H-1-benzenepyran-2- methanol) methane sulfosalt
A benzopyran, methylene technology, applied in the field of medicinal chemistry, can solve the problems of difficulty in mass production, expensive raw materials and reagents, complicated reaction operations and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1).6-fluoro-3,4-dihydro-2-oxiranyl-2H-1-benzopyran (1 shown in scheme 2) (3.0 grams, 0.016mol), benzylamine (8.3 gram, 0.08mol), isopropanol 100ml, add in the reaction vessel, reflux reaction 2 hours. After stopping the reaction, the solvent was distilled off to obtain a white solid. Recrystallization gave pure white solid 1-[6-fluoro-3,4-dihydro-2H-1-benzopyran-2-yl]-2-benzylamino-ethanol (shown in Scheme 2) ( Yield 85%), mp 117~118 ℃.
[0029] (2).6-fluoro-3,4-dihydro-2-oxirane group-2H-1-benzopyran (1 shown in scheme 2) (8.0 grams, 40mmol), the ammoniacal liquor of 25wt% ( 0.27 grams, 4.0mmol), methanol 800ml, after reflux reaction for 2 hours. The solvent was evaporated to obtain a yellow thick substance. Silica gel column purification, using dichloromethane and methanol as eluent. After evaporation of the solvent, white solid 1-[6-fluoro-3,4-dihydro-2H-1-benzopyran-2-yl]-2-amino-ethanol (4 shown in Scheme 2) was obtained (yield Rate 78%), mp 119 ~ 112 ℃.
...
Embodiment 2
[0035] (1).6-fluoro-3,4-dihydro-2-oxiranyl-2H-1-benzopyran (1 shown in scheme 2) (7.5 grams, 0.04mol), benzylamine (0.83 gram, 0.008mol), tetrahydrofuran 20ml, add in the reaction container, reflux reaction 2 hours. After stopping the reaction, the solvent was distilled off to obtain a white solid. Recrystallization gave pure white solid 1-[6-fluoro-3,4-dihydro-2H-1-benzopyran-2-yl]-2-benzylamino-ethanol (shown in Scheme 2) ( Yield 85%), mp 117~118 ℃.
[0036] (2).6-fluoro-3,4-dihydro-2-oxiranyl-2H-1-benzopyran (1 shown in Scheme 2) (1.6 g, 8.0 mmol), 25% ammonia (0.54 g, 8.0 mmol), butanol 70 ml, after reflux reaction for 2 hours. The solvent was evaporated to obtain a yellow thick substance. Silica gel column purification, using dichloromethane and methanol as eluent. After evaporation of the solvent, white solid 1-[6-fluoro-3,4-dihydro-2H-1-benzopyran-2-yl]-2-amino-ethanol (4 shown in Scheme 2) was obtained (yield Rate 78%), mp 119 ~ 112 ℃.
[0037] or
[0038] The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com