Transdermal absorption preparation
A preparation and liquid preparation technology, applied in the field of new percutaneous absorption-promoting compositions and percutaneous absorption preparations, can solve problems such as strong skin irritation, drug effectiveness, and damage to drug stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
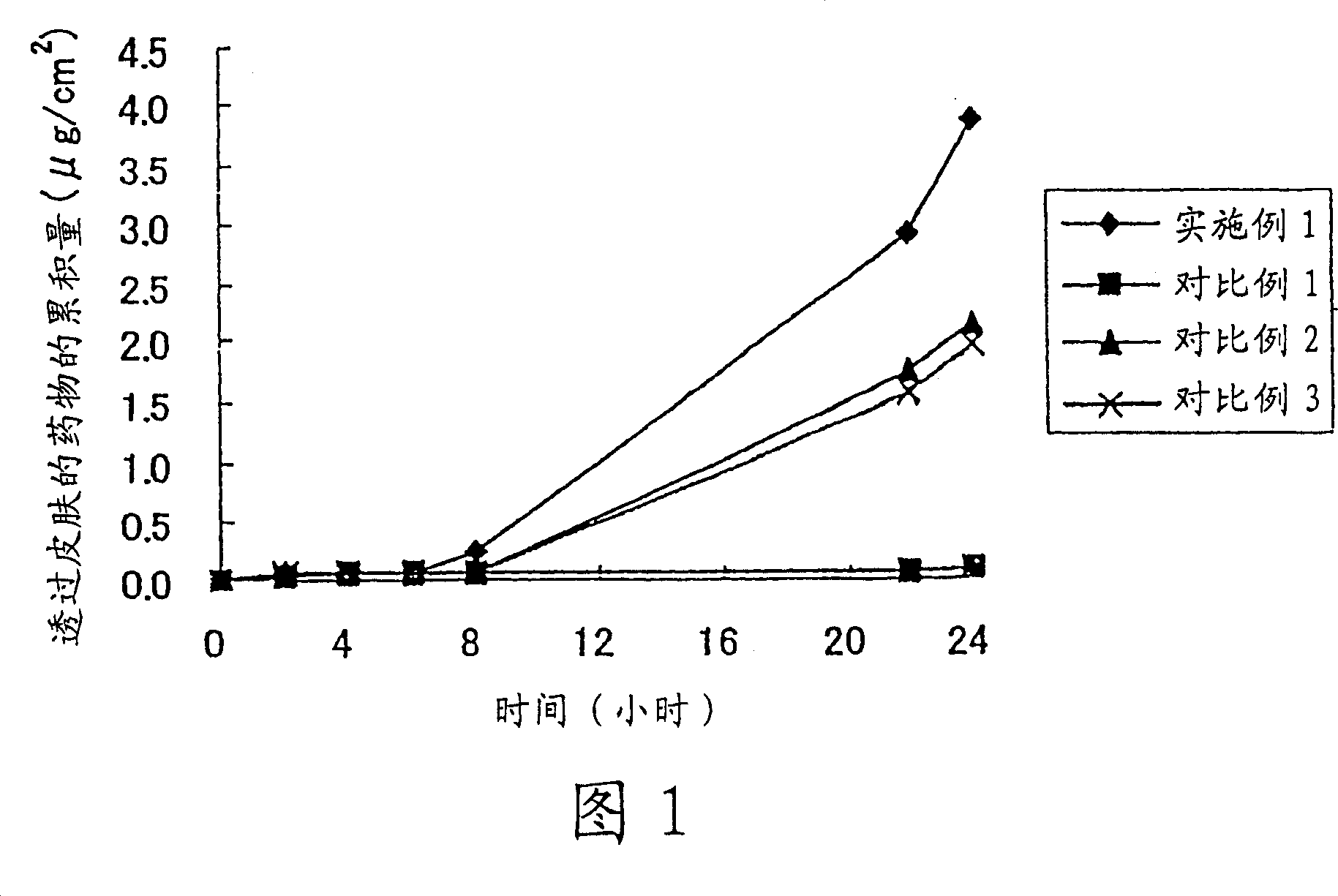
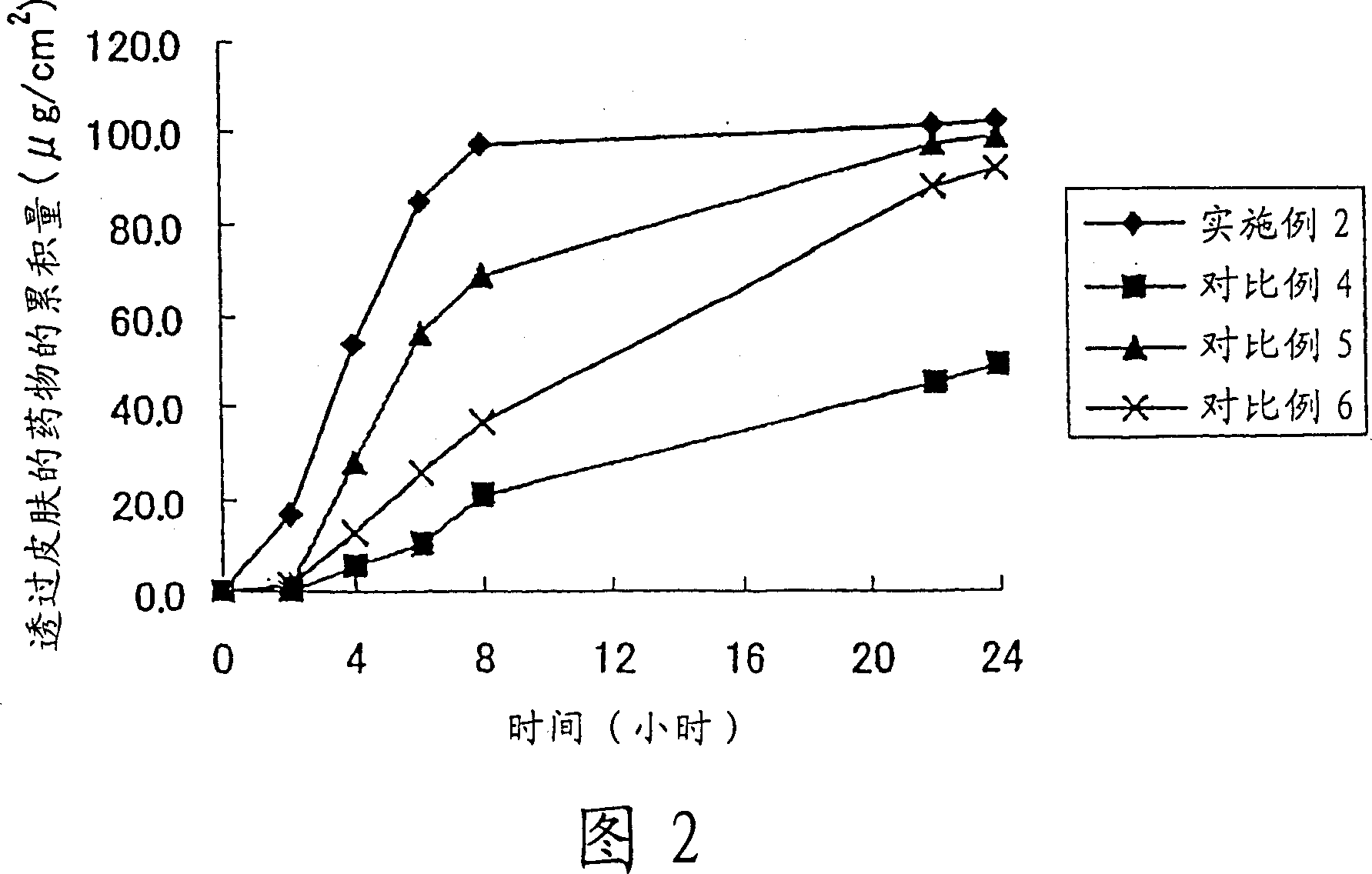
Image
Examples
Embodiment 1
[0043] Propylene glycol monocaprylate, polyoxyethylene (2) lauryl ether, and propylene glycol were mixed in a weight ratio of 1:1:8 to obtain a transdermal absorption-promoting composition. 1 part by weight of loperamide hydrochloride was dissolved in 99 parts by weight of the percutaneous absorption-promoting composition to prepare a homogeneous solution.
Embodiment 2
[0055] 1 part by weight of lidocaine was dissolved in 10 parts by weight of propylene glycol. Add and dissolve 4 parts by weight of propylene glycol monocaprylate, 4 parts by weight of polyoxyethylene (2) lauryl ether, 5 parts by weight of sorbitan sesquioleate and 4 parts by weight of paraffin wax in the solution. Then, 1.5 parts by weight of light silica and 70.5 parts by weight of white petrolatum were added, thereby preparing an ointment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com