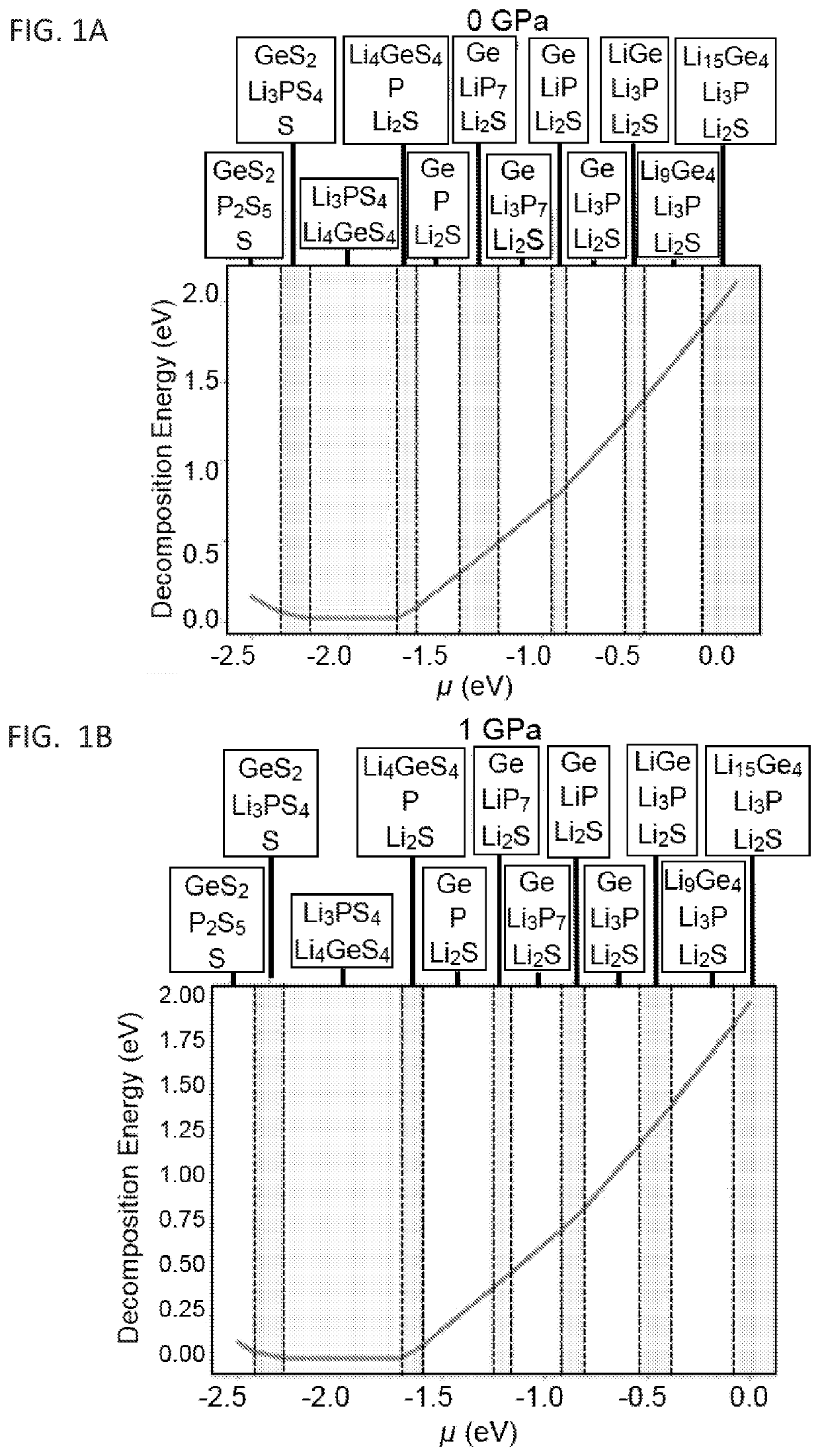
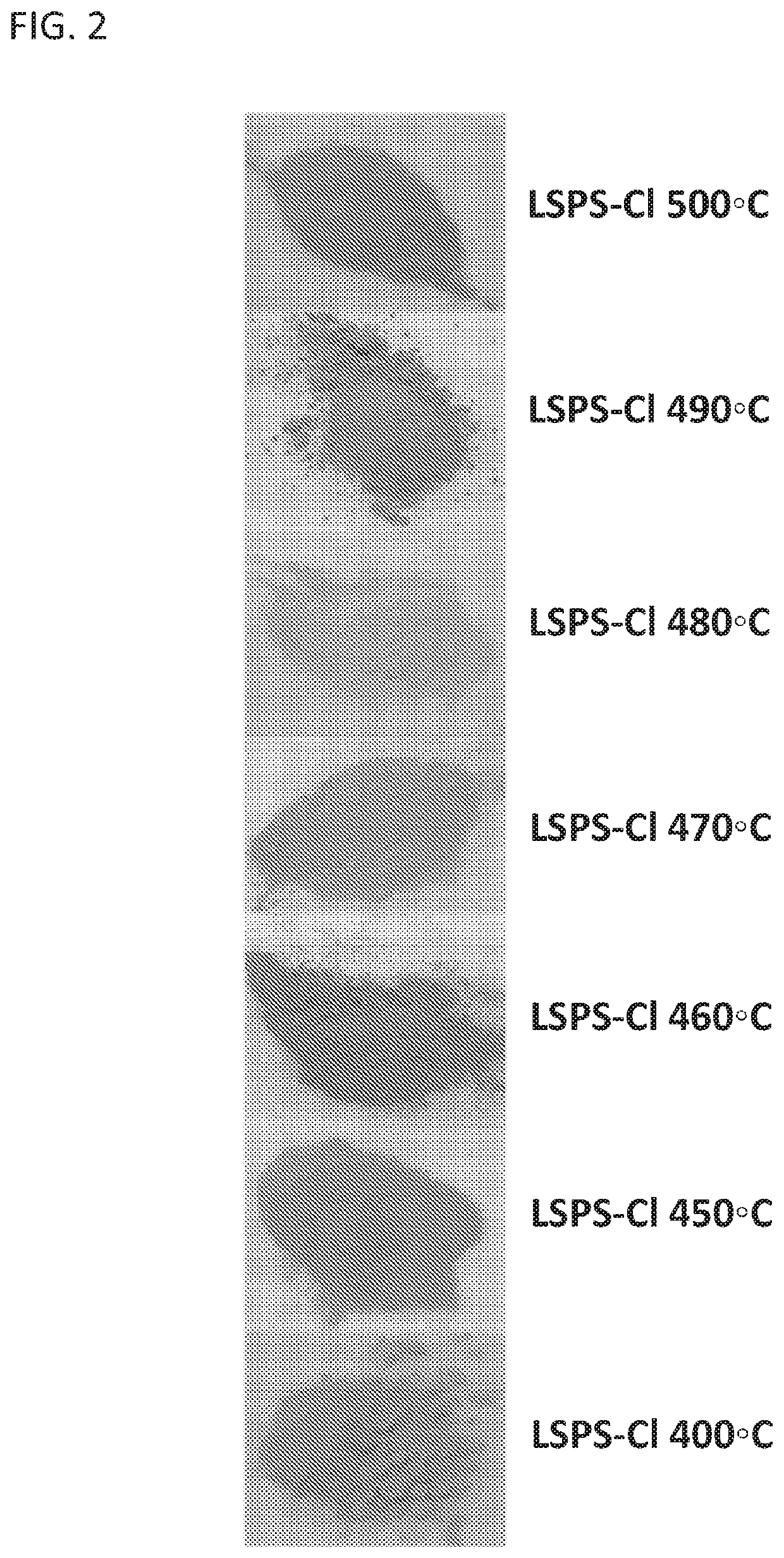
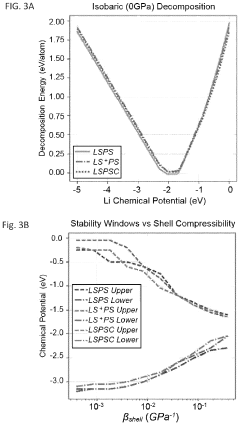
Standard Testing Protocols For Chloride SSE Performance And Durability
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chloride SSE Testing Background and Objectives
Solid-state electrolytes (SSEs) have emerged as a promising alternative to liquid electrolytes in battery technology, offering enhanced safety and potential for higher energy density. Among various SSE types, chloride-based solid-state electrolytes have gained significant attention due to their high ionic conductivity and relatively low cost. The development of standardized testing protocols for chloride SSE performance and durability represents a critical step toward the commercialization of solid-state batteries.
The evolution of chloride-based SSEs can be traced back to the early 2000s, with significant breakthroughs occurring in the 2010s when researchers discovered several chloride-based materials with room temperature ionic conductivities comparable to liquid electrolytes. Notable milestones include the discovery of Li3YCl6 and Li3InCl6 systems, which demonstrated ionic conductivities exceeding 1 mS/cm at room temperature. These developments catalyzed extensive research into chloride-based SSEs as viable candidates for next-generation energy storage solutions.
Despite promising advances, the field has been hampered by inconsistent testing methodologies across research institutions and industry players. The lack of standardized protocols has led to difficulties in comparing results across studies, hindering technological progress and commercial adoption. Current testing approaches vary widely in terms of sample preparation, measurement conditions, and durability assessment parameters, creating significant challenges for technology evaluation and benchmarking.
The primary technical objective for chloride SSE testing standardization is to establish universally accepted protocols that accurately evaluate key performance metrics, including ionic conductivity, electrochemical stability windows, interfacial resistance, and long-term durability under various operating conditions. These protocols must address the unique challenges posed by chloride-based materials, such as their moisture sensitivity, potential for chlorine gas evolution, and interfacial instability with electrode materials.
Additionally, standardized testing must account for the multifaceted nature of SSE performance, which encompasses not only basic conductivity measurements but also mechanical properties, chemical stability, and compatibility with manufacturing processes. The protocols should enable meaningful comparison between different chloride SSE formulations and provide insights into their practical viability for commercial battery applications.
Looking forward, the development of these standardized testing protocols aims to accelerate innovation in chloride-based SSEs by providing clear benchmarks for performance evaluation. This will facilitate more efficient research and development cycles, enable more accurate technology forecasting, and ultimately support the transition from laboratory-scale demonstrations to commercial solid-state battery products incorporating chloride-based electrolytes.
The evolution of chloride-based SSEs can be traced back to the early 2000s, with significant breakthroughs occurring in the 2010s when researchers discovered several chloride-based materials with room temperature ionic conductivities comparable to liquid electrolytes. Notable milestones include the discovery of Li3YCl6 and Li3InCl6 systems, which demonstrated ionic conductivities exceeding 1 mS/cm at room temperature. These developments catalyzed extensive research into chloride-based SSEs as viable candidates for next-generation energy storage solutions.
Despite promising advances, the field has been hampered by inconsistent testing methodologies across research institutions and industry players. The lack of standardized protocols has led to difficulties in comparing results across studies, hindering technological progress and commercial adoption. Current testing approaches vary widely in terms of sample preparation, measurement conditions, and durability assessment parameters, creating significant challenges for technology evaluation and benchmarking.
The primary technical objective for chloride SSE testing standardization is to establish universally accepted protocols that accurately evaluate key performance metrics, including ionic conductivity, electrochemical stability windows, interfacial resistance, and long-term durability under various operating conditions. These protocols must address the unique challenges posed by chloride-based materials, such as their moisture sensitivity, potential for chlorine gas evolution, and interfacial instability with electrode materials.
Additionally, standardized testing must account for the multifaceted nature of SSE performance, which encompasses not only basic conductivity measurements but also mechanical properties, chemical stability, and compatibility with manufacturing processes. The protocols should enable meaningful comparison between different chloride SSE formulations and provide insights into their practical viability for commercial battery applications.
Looking forward, the development of these standardized testing protocols aims to accelerate innovation in chloride-based SSEs by providing clear benchmarks for performance evaluation. This will facilitate more efficient research and development cycles, enable more accurate technology forecasting, and ultimately support the transition from laboratory-scale demonstrations to commercial solid-state battery products incorporating chloride-based electrolytes.
Market Requirements for Chloride SSE Performance Standards
The solid-state battery market is experiencing rapid growth, with projections indicating a compound annual growth rate exceeding 30% through 2030. Within this expanding market, chloride solid-state electrolytes (SSEs) have emerged as promising candidates for next-generation battery technologies due to their high ionic conductivity and potential for improved safety profiles compared to conventional liquid electrolytes.
Industry stakeholders, including battery manufacturers, automotive OEMs, and electronics companies, are increasingly demanding standardized testing protocols for chloride SSEs to facilitate meaningful performance comparisons and accelerate commercialization efforts. The absence of universally accepted performance standards has created significant market inefficiencies, with companies developing proprietary testing methodologies that yield incomparable results.
Primary market requirements for chloride SSE performance standards center around five key performance indicators: ionic conductivity, electrochemical stability window, mechanical properties, chemical stability, and long-term durability. Battery manufacturers require precise conductivity measurements at various operating temperatures (typically -20°C to 80°C) to ensure reliable performance across diverse applications and environments.
Automotive industry stakeholders emphasize the need for standardized safety testing protocols, particularly regarding chloride SSE stability against lithium metal anodes and high-voltage cathodes. These standards must address dendrite formation resistance and interface stability under various charge-discharge conditions to meet stringent vehicle safety requirements.
Consumer electronics manufacturers prioritize standards that evaluate chloride SSE performance under rapid charging conditions and after numerous cycles, reflecting real-world usage patterns. Additionally, they require protocols that assess SSE behavior under mechanical stress to ensure device durability.
Energy storage system developers demand standards that evaluate chloride SSE performance during extended storage periods and under varying environmental conditions, including humidity exposure and temperature fluctuations. These requirements stem from the need for grid storage solutions with 10+ year operational lifespans.
Regulatory bodies and insurance companies are increasingly requesting standardized safety certification protocols specific to chloride SSE technologies. This market demand is driven by the need to establish appropriate risk assessment frameworks and insurance models for emerging solid-state battery technologies.
Material suppliers require benchmarking standards to position their products effectively in the market and provide customers with comparable performance metrics. This demand is particularly strong among startups developing novel chloride SSE formulations seeking to validate their technology against established alternatives.
Industry stakeholders, including battery manufacturers, automotive OEMs, and electronics companies, are increasingly demanding standardized testing protocols for chloride SSEs to facilitate meaningful performance comparisons and accelerate commercialization efforts. The absence of universally accepted performance standards has created significant market inefficiencies, with companies developing proprietary testing methodologies that yield incomparable results.
Primary market requirements for chloride SSE performance standards center around five key performance indicators: ionic conductivity, electrochemical stability window, mechanical properties, chemical stability, and long-term durability. Battery manufacturers require precise conductivity measurements at various operating temperatures (typically -20°C to 80°C) to ensure reliable performance across diverse applications and environments.
Automotive industry stakeholders emphasize the need for standardized safety testing protocols, particularly regarding chloride SSE stability against lithium metal anodes and high-voltage cathodes. These standards must address dendrite formation resistance and interface stability under various charge-discharge conditions to meet stringent vehicle safety requirements.
Consumer electronics manufacturers prioritize standards that evaluate chloride SSE performance under rapid charging conditions and after numerous cycles, reflecting real-world usage patterns. Additionally, they require protocols that assess SSE behavior under mechanical stress to ensure device durability.
Energy storage system developers demand standards that evaluate chloride SSE performance during extended storage periods and under varying environmental conditions, including humidity exposure and temperature fluctuations. These requirements stem from the need for grid storage solutions with 10+ year operational lifespans.
Regulatory bodies and insurance companies are increasingly requesting standardized safety certification protocols specific to chloride SSE technologies. This market demand is driven by the need to establish appropriate risk assessment frameworks and insurance models for emerging solid-state battery technologies.
Material suppliers require benchmarking standards to position their products effectively in the market and provide customers with comparable performance metrics. This demand is particularly strong among startups developing novel chloride SSE formulations seeking to validate their technology against established alternatives.
Current Testing Limitations and Technical Barriers
Despite significant advancements in chloride solid-state electrolyte (SSE) research, standardized testing protocols for performance and durability remain underdeveloped. Current testing methodologies exhibit considerable variability across research institutions, making direct comparison of results challenging. This inconsistency stems from the absence of universally accepted testing parameters, including temperature ranges, pressure conditions, and cycling protocols.
A major limitation is the lack of accelerated aging tests specifically designed for chloride SSEs. Unlike liquid electrolytes, solid electrolytes experience unique degradation mechanisms that current testing frameworks fail to adequately capture. The interface stability between chloride SSEs and electrodes represents another critical testing challenge, as conventional methods often cannot accurately measure the complex electrochemical reactions occurring at these boundaries.
Environmental control during testing presents significant technical barriers. Chloride-based SSEs are notably sensitive to moisture and oxygen exposure, yet many laboratories lack standardized environmental chambers capable of maintaining consistent testing conditions. This variability introduces uncontrolled factors that compromise result reliability and reproducibility across different research groups.
The mechanical property evaluation of chloride SSEs faces substantial limitations. Current testing protocols inadequately address the relationship between mechanical stress and electrochemical performance, despite this being crucial for practical applications. Techniques for measuring interfacial resistance evolution during cycling remain primitive, limiting understanding of long-term performance degradation mechanisms.
Analytical techniques for post-test characterization also present barriers. The detection sensitivity for trace impurities that may catalyze degradation reactions in chloride SSEs is often insufficient. Additionally, in-situ characterization methods capable of monitoring structural and compositional changes during operation are underdeveloped, hindering real-time degradation analysis.
Scale-up testing represents another significant gap. Most current protocols are designed for small laboratory samples, failing to address the unique challenges that emerge at commercially relevant scales. This disconnect between laboratory testing and industrial requirements creates uncertainty regarding the practical viability of promising materials.
Computational modeling tools to complement experimental testing remain limited. The absence of validated models that can accurately predict long-term degradation behavior forces researchers to rely heavily on time-consuming experimental approaches, slowing innovation cycles and technology development timelines.
A major limitation is the lack of accelerated aging tests specifically designed for chloride SSEs. Unlike liquid electrolytes, solid electrolytes experience unique degradation mechanisms that current testing frameworks fail to adequately capture. The interface stability between chloride SSEs and electrodes represents another critical testing challenge, as conventional methods often cannot accurately measure the complex electrochemical reactions occurring at these boundaries.
Environmental control during testing presents significant technical barriers. Chloride-based SSEs are notably sensitive to moisture and oxygen exposure, yet many laboratories lack standardized environmental chambers capable of maintaining consistent testing conditions. This variability introduces uncontrolled factors that compromise result reliability and reproducibility across different research groups.
The mechanical property evaluation of chloride SSEs faces substantial limitations. Current testing protocols inadequately address the relationship between mechanical stress and electrochemical performance, despite this being crucial for practical applications. Techniques for measuring interfacial resistance evolution during cycling remain primitive, limiting understanding of long-term performance degradation mechanisms.
Analytical techniques for post-test characterization also present barriers. The detection sensitivity for trace impurities that may catalyze degradation reactions in chloride SSEs is often insufficient. Additionally, in-situ characterization methods capable of monitoring structural and compositional changes during operation are underdeveloped, hindering real-time degradation analysis.
Scale-up testing represents another significant gap. Most current protocols are designed for small laboratory samples, failing to address the unique challenges that emerge at commercially relevant scales. This disconnect between laboratory testing and industrial requirements creates uncertainty regarding the practical viability of promising materials.
Computational modeling tools to complement experimental testing remain limited. The absence of validated models that can accurately predict long-term degradation behavior forces researchers to rely heavily on time-consuming experimental approaches, slowing innovation cycles and technology development timelines.
Established Testing Protocols for Chloride SSE Evaluation
01 Composition and structure of chloride-based solid-state electrolytes
Chloride-based solid-state electrolytes (SSEs) can be formulated with various compositions to enhance their performance and durability. These electrolytes typically contain metal chlorides such as lithium chloride combined with other elements to form stable crystalline structures. The specific composition and crystal structure significantly influence ionic conductivity and electrochemical stability. Optimization of the chloride SSE composition can lead to improved performance in solid-state batteries by enhancing ion transport pathways while maintaining structural integrity.- Composition and structure of chloride-based SSEs: Chloride-based solid-state electrolytes can be formulated with various compositions to enhance performance and durability. These electrolytes typically contain metal chlorides such as lithium chloride, combined with other components to form stable crystalline or amorphous structures. The specific composition and crystal structure significantly impact ionic conductivity and electrochemical stability. Optimization of the chloride ratio and incorporation of dopants can improve the overall performance of these electrolytes in battery applications.
- Interface stability and degradation mechanisms: The interface between chloride solid-state electrolytes and electrodes is critical for long-term durability. These interfaces are susceptible to chemical and electrochemical degradation, leading to increased resistance and decreased battery performance over time. Mechanisms of degradation include formation of interphases, chloride ion migration, and reactions with electrode materials. Understanding and mitigating these degradation processes is essential for improving the cycle life and reliability of chloride-based solid-state batteries.
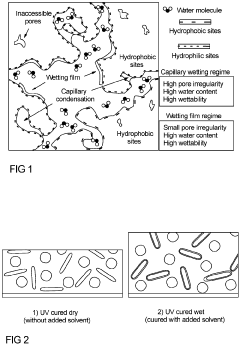
- Moisture sensitivity and protection strategies: Chloride-based solid-state electrolytes are highly sensitive to moisture, which can lead to significant degradation of their performance and durability. Exposure to ambient humidity can cause hydrolysis reactions, structural changes, and loss of ionic conductivity. Various protection strategies have been developed, including hydrophobic coatings, encapsulation techniques, and moisture-resistant additives. These approaches aim to maintain the integrity and performance of chloride SSEs under practical operating conditions.
- Temperature effects and thermal stability: The performance and durability of chloride solid-state electrolytes are significantly influenced by temperature. These materials often exhibit improved ionic conductivity at elevated temperatures but may suffer from thermal decomposition or phase transitions that affect long-term stability. Engineering chloride SSEs with enhanced thermal stability across a wide temperature range is crucial for their application in various environments. Thermal management strategies and temperature-resistant formulations help maintain consistent performance under thermal cycling conditions.
- Manufacturing processes and scalability: The manufacturing processes used to produce chloride solid-state electrolytes significantly impact their performance and durability. Techniques such as melt processing, solution methods, and solid-state reactions yield materials with different microstructures and defect concentrations. Optimized processing conditions can reduce impurities and enhance homogeneity, leading to improved ionic conductivity and mechanical properties. Scalable manufacturing approaches that maintain quality while enabling mass production are essential for commercial viability of chloride SSE-based batteries.
02 Interface stability and degradation mechanisms
The interface between chloride solid-state electrolytes and electrodes is critical for battery performance and durability. Chloride SSEs often face challenges related to interfacial resistance and chemical instability when in contact with electrode materials. Degradation mechanisms include formation of resistive interphases, chloride ion migration, and structural changes under operating conditions. Understanding and mitigating these interface issues through protective coatings, buffer layers, or compositional modifications can significantly improve the cycle life and performance stability of chloride-based solid-state batteries.Expand Specific Solutions03 Ionic conductivity enhancement strategies
Enhancing ionic conductivity is crucial for improving the performance of chloride solid-state electrolytes. Various strategies can be employed, including doping with aliovalent ions, creating defect structures, and optimizing grain boundaries. The incorporation of specific dopants can create additional vacancies in the crystal lattice, facilitating faster ion transport. Processing techniques such as controlled sintering and pressure application can also reduce grain boundary resistance, leading to higher overall ionic conductivity while maintaining mechanical integrity and electrochemical stability.Expand Specific Solutions04 Environmental stability and moisture resistance
Chloride-based solid-state electrolytes often exhibit sensitivity to environmental factors, particularly moisture, which can significantly impact their durability and performance. Exposure to humidity can lead to hydrolysis reactions, phase transformations, and degradation of electrochemical properties. Strategies to improve environmental stability include encapsulation techniques, hydrophobic surface modifications, and compositional adjustments to create more moisture-resistant structures. These approaches help maintain the integrity and performance of chloride SSEs under various operating conditions and extend their practical service life.Expand Specific Solutions05 Manufacturing processes and scalability
The manufacturing processes for chloride solid-state electrolytes significantly influence their performance characteristics and durability. Techniques such as cold sintering, mechanochemical synthesis, and solution-based methods can be optimized to control grain size, density, and homogeneity. Processing parameters including temperature, pressure, and atmosphere during synthesis and fabrication play crucial roles in determining the final properties of the electrolyte. Scalable manufacturing approaches that maintain quality while enabling cost-effective production are essential for the commercial viability of chloride SSE-based battery technologies.Expand Specific Solutions
Leading Organizations in SSE Testing Protocol Development
The chloride solid-state electrolyte (SSE) performance testing market is currently in an early growth phase, characterized by increasing research activity but limited standardization. Market size is expanding as battery technology advances, with projections showing significant growth potential as SSEs become critical for next-generation energy storage solutions. Technologically, the field remains in development with varying maturity levels across different testing protocols. Leading companies like FUJIFILM, Regeneron Pharmaceuticals, and Oxford University Innovation are advancing proprietary testing methodologies, while research institutions such as Nanyang Technological University and Osaka University contribute fundamental research. Chinese organizations including State Grid and various universities are increasingly active in establishing regional testing standards, creating a competitive landscape where academic-industrial partnerships are becoming essential for establishing globally accepted testing protocols.
Sobute New Materials Co., Ltd.
Technical Solution: Sobute New Materials has developed specialized testing protocols for chloride solid-state electrolytes (SSEs) with particular focus on construction and infrastructure applications. Their approach integrates electrochemical performance testing with durability assessments under realistic environmental conditions. Sobute's protocols include standardized chloride ion penetration tests using both electrical conductivity methods and accelerated migration techniques under applied electric fields. They've established multi-stage aging protocols that expose chloride SSEs to cyclic wetting/drying with chloride solutions of varying concentrations (0.1M to 5.0M) while monitoring performance degradation. Their testing framework incorporates mechanical integrity evaluations before and after chloride exposure, including compressive strength, flexural strength, and microstructural analysis using scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX). Sobute has also developed specialized protocols for evaluating the protective performance of chloride SSEs when applied to reinforced concrete, measuring corrosion potential, corrosion current density, and time-to-corrosion initiation under accelerated conditions.
Strengths: Comprehensive protocols specifically designed for construction applications with direct correlation to real-world performance requirements. Their methods combine electrochemical, physical, and mechanical testing to provide holistic performance assessment. Weaknesses: Testing protocols may be overly specialized for construction applications, potentially limiting their applicability to other fields such as energy storage where different performance metrics might be more relevant.
Nanjing Bote New Materials Co. Ltd.
Technical Solution: Nanjing Bote New Materials has developed specialized testing protocols for chloride solid-state electrolytes focusing on industrial applicability and quality control. Their approach includes standardized ionic conductivity measurements using AC impedance spectroscopy across a wide temperature range (-20°C to 100°C) to evaluate performance under various operating conditions. The company has established accelerated environmental testing chambers that simulate multiple degradation factors simultaneously, including temperature fluctuations, humidity cycling, and mechanical stress. Their protocols incorporate electrochemical stability window determination using cyclic voltammetry with standardized electrode configurations and scan rates. Nanjing Bote's testing methodology includes long-term cycling tests (>1000 cycles) with intermittent characterization to track performance degradation over time. They've also developed specialized interfacial resistance measurement techniques to evaluate the compatibility between chloride SSEs and various electrode materials, which is critical for predicting long-term battery performance and durability.
Strengths: Industry-focused protocols designed for manufacturing quality control with emphasis on reproducibility and throughput. Their methods include practical performance metrics directly relevant to commercial applications. Weaknesses: Testing protocols may prioritize production efficiency over fundamental scientific understanding of degradation mechanisms, potentially missing subtle failure modes that emerge only under specific conditions.
Critical Parameters and Measurement Techniques
Solid state electrolytes and methods of production thereof
PatentActiveUS20200350618A1
Innovation
- A solid state electrolyte with a core-shell morphology is developed, incorporating an alkali metal and sulfide, where the shell enhances stability by reducing core expansion during electrical cycling, and is fabricated through a method involving mixing sulfur, phosphorus, and alkali metal sources, followed by annealing to achieve a composition like Li9.54Si1.74P1.44S11.7Cl0.3.
Polymer based solid-state electrolyte (SSE) for deposition on an ag/agcl reference electrode
PatentPendingEP4336177A1
Innovation
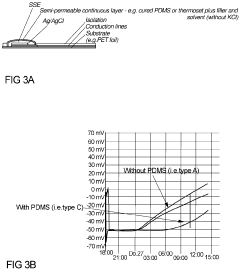
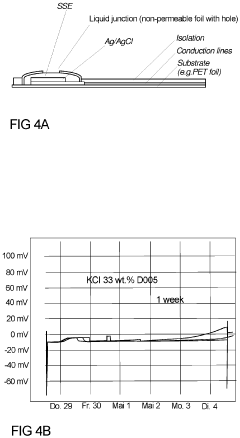
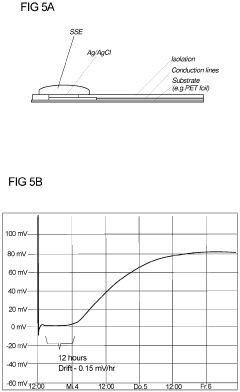
- A solid-state electrolyte comprising a curable polymer matrix with amphiphilic mineral fillers and an organic solvent, optimized for rapid hydration and structural stability, is deposited on the Ag/AgCl electrode, controlling porosity and preventing chloride leakage through the use of low-vapour pressure solvents and specific filler materials like talc.
International Standards Harmonization
The harmonization of international standards for chloride solid-state electrolyte (SSE) testing represents a critical challenge in the global advancement of solid-state battery technology. Currently, significant disparities exist between testing protocols established by organizations such as the International Electrotechnical Commission (IEC), ASTM International, and various regional standards bodies including Japan's JIS, China's GB standards, and Europe's EN standards. These inconsistencies create barriers to technology transfer, market access, and comparative performance evaluation.
A comprehensive analysis of existing standards reveals that chloride SSE testing protocols differ substantially in key parameters including sample preparation methods, testing conditions, and performance metrics. For instance, the temperature ranges specified for durability testing vary from -20°C to 80°C in some standards to -40°C to 125°C in others, creating incomparable results across different certification systems.
The International Organization for Standardization (ISO) has initiated efforts through Technical Committee 333 to develop unified testing frameworks for solid-state battery components, with working group WG4 specifically addressing electrolyte materials. This initiative aims to establish consensus on critical testing parameters including ionic conductivity measurement techniques, electrochemical stability windows, and accelerated aging protocols specific to chloride-based SSEs.
Industry consortia are playing an increasingly important role in standards harmonization. The Battery Standards Consortium (BSC), comprising 27 companies across North America, Europe, and Asia, has proposed a unified testing framework that addresses chloride SSE-specific challenges including moisture sensitivity protocols and interfacial resistance measurement methodologies. Their proposal has gained traction among major battery manufacturers and is being considered for adoption by formal standards organizations.
Academic institutions are contributing to harmonization efforts through round-robin testing initiatives. The International Solid-State Battery Initiative (ISSBI), involving 14 universities across 8 countries, has completed comparative analyses of different testing protocols, identifying key discrepancies and recommending standardized approaches for chloride SSE evaluation. Their findings suggest that harmonized protocols could reduce inter-laboratory variation by up to 65%.
Regulatory bodies are increasingly recognizing the need for harmonized standards to facilitate market access and ensure safety compliance. The U.S. Department of Energy's Federal Consortium for Advanced Batteries has identified standards harmonization as a priority area in its national battery blueprint, while the European Commission has incorporated similar objectives in its Strategic Action Plan on Batteries.
A comprehensive analysis of existing standards reveals that chloride SSE testing protocols differ substantially in key parameters including sample preparation methods, testing conditions, and performance metrics. For instance, the temperature ranges specified for durability testing vary from -20°C to 80°C in some standards to -40°C to 125°C in others, creating incomparable results across different certification systems.
The International Organization for Standardization (ISO) has initiated efforts through Technical Committee 333 to develop unified testing frameworks for solid-state battery components, with working group WG4 specifically addressing electrolyte materials. This initiative aims to establish consensus on critical testing parameters including ionic conductivity measurement techniques, electrochemical stability windows, and accelerated aging protocols specific to chloride-based SSEs.
Industry consortia are playing an increasingly important role in standards harmonization. The Battery Standards Consortium (BSC), comprising 27 companies across North America, Europe, and Asia, has proposed a unified testing framework that addresses chloride SSE-specific challenges including moisture sensitivity protocols and interfacial resistance measurement methodologies. Their proposal has gained traction among major battery manufacturers and is being considered for adoption by formal standards organizations.
Academic institutions are contributing to harmonization efforts through round-robin testing initiatives. The International Solid-State Battery Initiative (ISSBI), involving 14 universities across 8 countries, has completed comparative analyses of different testing protocols, identifying key discrepancies and recommending standardized approaches for chloride SSE evaluation. Their findings suggest that harmonized protocols could reduce inter-laboratory variation by up to 65%.
Regulatory bodies are increasingly recognizing the need for harmonized standards to facilitate market access and ensure safety compliance. The U.S. Department of Energy's Federal Consortium for Advanced Batteries has identified standards harmonization as a priority area in its national battery blueprint, while the European Commission has incorporated similar objectives in its Strategic Action Plan on Batteries.
Environmental Impact Assessment of Testing Procedures
The environmental impact of testing protocols for chloride solid-state electrolytes (SSEs) represents a critical yet often overlooked aspect of battery technology development. Current testing procedures typically involve multiple chemical processes that generate waste materials, consume energy, and potentially release harmful substances into the environment. Laboratory testing of chloride SSEs frequently utilizes solvents such as dimethyl carbonate and ethylene carbonate, which can contribute to air pollution and pose disposal challenges when released as volatile organic compounds (VOCs).
Energy consumption during testing constitutes another significant environmental concern. Long-duration cycling tests and temperature-controlled environments require continuous power supply, often from non-renewable sources. Quantitative assessments indicate that a standard 1000-cycle durability test for chloride SSEs can consume between 50-75 kWh of electricity, equivalent to the monthly energy usage of some household appliances.
Water usage in testing protocols also merits environmental consideration. Cleaning procedures, sample preparation, and certain electrochemical measurements require purified water, with a typical testing series consuming 20-30 liters. This becomes particularly relevant in regions facing water scarcity challenges, where laboratory water usage competes with other essential needs.
The disposal of test materials presents additional environmental challenges. Used chloride SSEs may contain lithium, aluminum, and other metals that require specialized handling procedures. Current estimates suggest that approximately 85% of laboratory test materials end up in general waste streams rather than being properly recycled, highlighting a significant gap in sustainable laboratory practices.
Recent innovations have begun addressing these environmental concerns through the development of greener testing protocols. These include miniaturized test cells that reduce material consumption by up to 60%, regenerative energy systems for cycling tests, and water recycling systems for laboratory operations. Several research institutions have implemented closed-loop testing systems that capture and reuse solvents, significantly reducing VOC emissions.
Standardization bodies are increasingly incorporating environmental impact metrics into their protocol development processes. The International Electrochemical Commission has recently established a working group focused specifically on sustainable testing methodologies for energy storage materials, with chloride SSEs identified as a priority area due to their growing importance in next-generation battery technologies.
Energy consumption during testing constitutes another significant environmental concern. Long-duration cycling tests and temperature-controlled environments require continuous power supply, often from non-renewable sources. Quantitative assessments indicate that a standard 1000-cycle durability test for chloride SSEs can consume between 50-75 kWh of electricity, equivalent to the monthly energy usage of some household appliances.
Water usage in testing protocols also merits environmental consideration. Cleaning procedures, sample preparation, and certain electrochemical measurements require purified water, with a typical testing series consuming 20-30 liters. This becomes particularly relevant in regions facing water scarcity challenges, where laboratory water usage competes with other essential needs.
The disposal of test materials presents additional environmental challenges. Used chloride SSEs may contain lithium, aluminum, and other metals that require specialized handling procedures. Current estimates suggest that approximately 85% of laboratory test materials end up in general waste streams rather than being properly recycled, highlighting a significant gap in sustainable laboratory practices.
Recent innovations have begun addressing these environmental concerns through the development of greener testing protocols. These include miniaturized test cells that reduce material consumption by up to 60%, regenerative energy systems for cycling tests, and water recycling systems for laboratory operations. Several research institutions have implemented closed-loop testing systems that capture and reuse solvents, significantly reducing VOC emissions.
Standardization bodies are increasingly incorporating environmental impact metrics into their protocol development processes. The International Electrochemical Commission has recently established a working group focused specifically on sustainable testing methodologies for energy storage materials, with chloride SSEs identified as a priority area due to their growing importance in next-generation battery technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!